A unique formyl iodoargentate exhibiting luminescent and photocurrent response properties†
Abstract
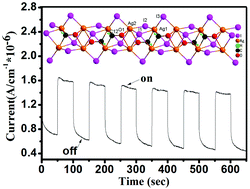
A decomposition and self-assembly reaction affords a novel formyl iodoargentate [H2L]n[Ag2I3(μ-CHO)]n (1, L = 2,6-bis(1-imdazoly)pyridine) with an unprecedented CHO− link mode, which provides the only example of iodoargentate incorporating the unstable formyl species under hydrothermal conditions. 1 exhibits luminescent and photocurrent response properties.


 Please wait while we load your content...
Please wait while we load your content...