gem-Dibromovinyl boron dipyrrins: synthesis, spectral properties and crystal structures†
Abstract
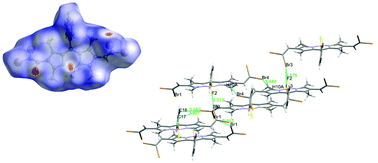
A family of new asymmetric and symmetric 1,3,7,9-tetramethyl-4,4-bora difluoro-diaza-s-indacene (BODIPY) derivatives, bearing gem-dibromovinyl substituents, was synthesized by the Corey–Fuchs olefination method. One or two gem-dibromovinyl moieties were attached at either the p-position of 5-phenyl, or the β-position of the pyrrole ring, directly or, through phenyl spacers. The assigned structures were supported by MS, NMR (1H, 13C, 19F), X-ray diffraction analysis and for some compounds 2D HSQC and 11B NMR as well as optical spectroscopy. Their absorption and fluorescence properties and solvatochromism in different solvents were investigated. The highest absorption and emission maxima were obtained for compounds having two gem-dibromovinyl groups attached directly or through the phenyl spacer. The best correlation (R-coefficient) between the solvent and spectral properties of the BODIPYs were obtained using the refractive index of the solvent. Although these compounds are structurally quite similar, their solid states show remarkable differences in the crystal system, clearly revealing two distinct patterns of gem-dibromovinyl orientation and torsion angles of the 5-phenyl ring and the indacene plane. Hirshfeld surface analysis data were used to visualize various intermolecular interactions.


 Please wait while we load your content...
Please wait while we load your content...