Open Access Article
Open Access ArticleCation exchange reversibly switches rotor speed and is monitored by a networked fluorescent reporter†
Merve S.
Özer
,
Indrajit
Paul
,
Abir
Goswami
and
Michael
Schmittel
 *
*
Center of Micro and Nanochemistry and Engineering, Organische Chemie I, Universität Siegen, Adolf-Reichwein-Str. 2, D-57068 Siegen, Germany. E-mail: schmittel@chemie.uni-siegen.de; Tel: +49(0) 2717404356
First published on 7th June 2019
Abstract
Framework 1, a freely rotating turnstile, is transformed by sequential metal ion addition into the coordination-based double-minimum rotors [M2(1)]2n+ that operate at 8 kHz (M = Zn2+; n = 2) and 30 kHz (M = Cu+; n = 1). In a network with the fluorescent receptor 2, the metal ion exchange at [M2(1)]2n+ and thus indirectly the rotor speed is reported by distinct fluorescence changes at 2.
Among the many types of molecular devices,1 thermally activated turnstiles2 and rotors3 allow us to study and comprehend the relationship between motion and smart molecular properties,4 as lately demonstrated in catalytic rotary machinery.5 The finding that product inhibition and catalytic activity directly depend on the machine speed5 suggests that one does not need a unidirectional motor for sophisticated effects in catalysis. Such results ask for a more systematic exploration of rotor systems with speed regulation.6
Despite recent advances,6 reversible toggling of rotational speed in rotors needs further exploration, in particular when the course of switching is to be reported by an optical signal. As shown by Hosseini, it is possible to control the motion of turnstiles by addition of metal ions (stop-go) and monitor the process by luminescence changes.2a,7 For instance, in a turnstile containing a bis-pyridyl terminated rotator and a tridentate-appended stator, drastic emission changes in the open vs. closed state (with Ag+ and Pd2+) allowed monitoring of the reversible ON/OFF switching of motion.2b
Implementation of speed regulation in rotors linked with optical monitoring requires matching of two functions in one device at the same time. Due to our expertise in networking devices8 we considered another approach, i.e. to link an external optical reporter to the rotor by chemical signalling. Such protocol constitutes an adaptable toolbox that could be applied to a variety of rotor and optical reporter systems.
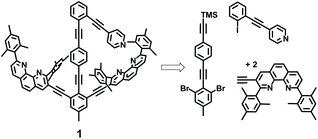
Here, we report on a networked system in which the two-state behaviour of rotor 1 that is reversibly regulated from slow to fast motion by addition/exchange of different metal ions is monitored by luminescence changes at a remote receptor 2 (Scheme 1). Our design was guided by the following considerations: intrinsic rotation within the framework 1 occurs about a tolane unit9 and thus should proceed without a sizable barrier as in a typical turnstile. The situation ought to change once metal ions (Zn2+ or Cu+) ions are added, since the pyridine terminals are expected to bind to the metal-loaded phenanthroline stations via HETPYP-I (HETeroleptic PYridine and Phenanthroline Metal)10 complexation. Now the arm is expected to exchange at defined speed between the two degenerate metal-loaded stations. The luminescent aza-crown ether 2 was designed for communication with 1 as we expected different emission for the free and cation-loaded receptor.
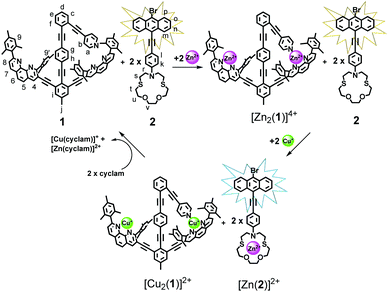 | ||
| Scheme 1 Communication between nanorotor 1 and reporter 2 upon sequential addition of Zn2+ and Cu+ ions. | ||
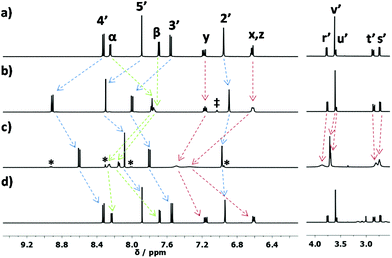
Before addressing the precise design (Scheme 1) of the rotor and luminescence reporter, we optimized the involved binding sites by studying various two-step self-sorting events. We observed that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blending of the shielded phenanthroline 3, aza-crown ether 4 and 4-iodopyridine (5) in presence of one equivalent of Zn2+ (Scheme 2) cleanly furnished the HETPYP-I10 complex [Zn(3)(5)]2+. In the 1H NMR, all phenanthroline protons of 3 were downfield shifted while those of pyridine, e.g. α-protons, were moved upfield from 8.25 to 7.78 ppm (Fig. 1a and b). The aza-crown ether 4 remained unaltered attesting a two-fold incomplete11 self-sorting. In the second self-sorting, addition of Cu+ triggered a translocation of Zn2+ from [Zn(3)(5)]2+ to aza-crown ether 4 to furnish [Zn(4)(5)]2+ while copper(I) coordinated to phenanthroline 3 (85%). Importantly, as the binding of Cu+ to phenanthroline 3 in CH2Cl2 (log
1 blending of the shielded phenanthroline 3, aza-crown ether 4 and 4-iodopyridine (5) in presence of one equivalent of Zn2+ (Scheme 2) cleanly furnished the HETPYP-I10 complex [Zn(3)(5)]2+. In the 1H NMR, all phenanthroline protons of 3 were downfield shifted while those of pyridine, e.g. α-protons, were moved upfield from 8.25 to 7.78 ppm (Fig. 1a and b). The aza-crown ether 4 remained unaltered attesting a two-fold incomplete11 self-sorting. In the second self-sorting, addition of Cu+ triggered a translocation of Zn2+ from [Zn(3)(5)]2+ to aza-crown ether 4 to furnish [Zn(4)(5)]2+ while copper(I) coordinated to phenanthroline 3 (85%). Importantly, as the binding of Cu+ to phenanthroline 3 in CH2Cl2 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 5.42 ± 0.10, see Fig. S63†) is stronger than that of Zn2+ (log
K = 5.42 ± 0.10, see Fig. S63†) is stronger than that of Zn2+ (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 4.58 ± 0.58, see Fig. S64†), copper(I) replaced zinc(II) at the phenanthroline binding site of 3 in CD2Cl2
K = 4.58 ± 0.58, see Fig. S64†), copper(I) replaced zinc(II) at the phenanthroline binding site of 3 in CD2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN (98
CD3CN (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). In the 1H NMR all proton signals of phenanthroline 3 were shifted upfield while both aromatic and aliphatic protons of aza-crown ether 4 showed downfield shifts as a result of zinc(II) binding (Fig. 1c). Finally, two equiv. of cyclam were added for removal of Cu+ and Zn2+ in order to regenerate the initial state represented by the free ligands 3, 4 and 5 (see NMR, Fig. 1d).
2). In the 1H NMR all proton signals of phenanthroline 3 were shifted upfield while both aromatic and aliphatic protons of aza-crown ether 4 showed downfield shifts as a result of zinc(II) binding (Fig. 1c). Finally, two equiv. of cyclam were added for removal of Cu+ and Zn2+ in order to regenerate the initial state represented by the free ligands 3, 4 and 5 (see NMR, Fig. 1d).
Guided by this model study, the design of the rotor and reporter was finalized (Scheme 1). In scaffold 1 the pyridine arm and shielded phenanthroline stations are covalently connected preventing the possibility of pyridine translocation in presence of Zn2+ and Cu+ as in the model self-sorting.
Scaffold 1 was synthesized through a series of Sonogashira couplings in 15 steps and characterized by NMR, ESI-MS and elemental analysis (Scheme 3, ESI†). Characteristic signals in the 1H NMR proved the formation of 1, e.g. peaks of the pyridine protons α-H and β-H appeared at 8.57 and 7.44 ppm in addition to distinctive phenanthroline proton signals. The MS peak at m/z = 1270.8 showed the expected mass for [1 + H]+.
For fluorescence monitoring, the aza-crown receptor in 2 was decorated with an anthracene unit. It was synthesized as described in the ESI† and characterized by spectroscopic methods and ESI-MS. After excitation at λ = 410 nm, the system 2 showed a bright yellow emission at λ = 552 nm as a result of intramolecular charge transfer (ICT). When the aza-crown ether part was coordinated to zinc(II), the fluorescence wavelength shifted to λ = 448 and 475 nm (vide infra).
Firstly, [Zn2(1)]4+ was prepared by mixing 1 and Zn(OTf)2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
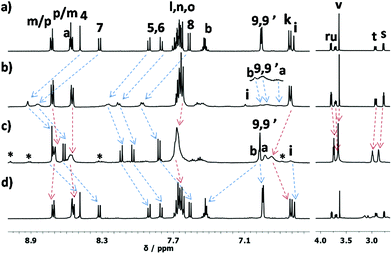
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. Phenanthroline protons 4-H to 8-H shifted downfield owing to zinc(II) ion coordination, while signals of pyridine protons α-H broadened and moved upfield, indicating that the zinc(II)-bound pyridine terminal was located in the shielding region of the mesityl groups of the zinc(II) phenanthroline stations (Fig. 2a and b). ESI-MS confirmed the formation by a peak at m/z = 858.0 representing [Zn2(1)(H2O)](OTf)22+. Its hydrodynamic radius was calculated to 11 Å by using D = 4.7 × 10−10 m2 s−1 from DOSY measurements (the DFT-computed theoretical radius is 9.6 Å). The single set of signals in the 1H NMR suggested rapid exchange of the pyridine arm between both zinc(II)-loaded phenanthroline stations. The kinetics of motion in [Zn2(1)]4+ was determined by a VT 1H NMR study from 25 °C to −75 °C in CD2Cl2/CD3CN (80
2 ratio. Phenanthroline protons 4-H to 8-H shifted downfield owing to zinc(II) ion coordination, while signals of pyridine protons α-H broadened and moved upfield, indicating that the zinc(II)-bound pyridine terminal was located in the shielding region of the mesityl groups of the zinc(II) phenanthroline stations (Fig. 2a and b). ESI-MS confirmed the formation by a peak at m/z = 858.0 representing [Zn2(1)(H2O)](OTf)22+. Its hydrodynamic radius was calculated to 11 Å by using D = 4.7 × 10−10 m2 s−1 from DOSY measurements (the DFT-computed theoretical radius is 9.6 Å). The single set of signals in the 1H NMR suggested rapid exchange of the pyridine arm between both zinc(II)-loaded phenanthroline stations. The kinetics of motion in [Zn2(1)]4+ was determined by a VT 1H NMR study from 25 °C to −75 °C in CD2Cl2/CD3CN (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) (Fig. 3a); the solvent mixture12 was required to keep the complex [Zn2(1)]4+ soluble. At 25 °C the single set of signals for the phenanthroline protons attested fast exchange, while rotational exchange slowed down visibly at −25 °C. Now two sets of signals emerged for phenanthroline proton 4-H, one resonating at 8.92 ppm for the pyridine → Zn2+ complexed site and the other at 8.81 ppm for the exclusively zinc(II)-loaded phenanthroline. The exchange frequency was determined as k25 = 8000 s−1 using WinD-NMR;13 the full set of activation data is depicted in Table 1.
20) (Fig. 3a); the solvent mixture12 was required to keep the complex [Zn2(1)]4+ soluble. At 25 °C the single set of signals for the phenanthroline protons attested fast exchange, while rotational exchange slowed down visibly at −25 °C. Now two sets of signals emerged for phenanthroline proton 4-H, one resonating at 8.92 ppm for the pyridine → Zn2+ complexed site and the other at 8.81 ppm for the exclusively zinc(II)-loaded phenanthroline. The exchange frequency was determined as k25 = 8000 s−1 using WinD-NMR;13 the full set of activation data is depicted in Table 1.
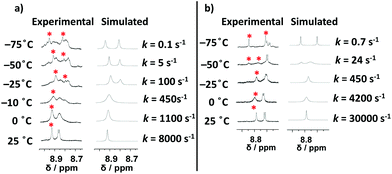 | ||
Fig. 3 Partial VT 1H NMR (600 MHz) at various temperatures of (a) [Zn2(1)]4+ in CD2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN (80 CD3CN (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), (b) [Cu2(1)]2+ in CD2Cl2. 20), (b) [Cu2(1)]2+ in CD2Cl2. | ||
| Nano-rotors | ΔH‡ (kJ mol−1) | ΔS‡ (J mol−1 K−1) | ΔG‡ (kJ mol−1) | k 25 (s−1) |
|---|---|---|---|---|
| [Zn2(1)]4+ | 53.4 ± 0.4 | 9.7 ± 2.0 | 50.5 | 8000 |
| [Cu2(1)]2+ | 50.4 ± 0.3 | 9.9 ± 1.2 | 47.4 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Similarly, the reaction of two equiv. of [Cu(CH3CN)4]PF6 with 1 furnished [Cu2(1)]2+ as proven by 1H NMR (Fig. 2a and c). Compared to nanorotor [Zn2(1)]4+, the phenanthroline protons were shifted upfield upon Cu+ coordination while the pyridine protons were slightly shifted downfield (Table 2). In the ESI-MS a signal at m/z = 719.5 (Fig. S58†) showed the formation of [Cu2(1)(CH3CN)]2+. The diffusion coefficient D = 4.4 × 10−10 m2 s−1 (DOSY) suggested a hydrodynamic radius of 12 Å (compare to 9.6 Å from the DFT-computed structure). As before, the 1H NMR showed fast exchange of the pyridine arm between both copper(I)-loaded phenanthroline stations. In the VT 1H NMR performed in CD2Cl2 from +25 to −75 °C (Fig. 3b), a splitting of protons 4-, 5-, 6-, 7-, 8-, 9- and 9′-H was observed. Kinetic parameters were calculated from the changes of proton 4-H in 1, which split into copper(I)-loaded (8.74 ppm) and pyridine → Cu+ complexed (8.85 ppm) phenanthroline stations at −50 °C. The exchange rate at room temperature was determined to k25 = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s−1 (Table 1).
000 s−1 (Table 1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN (96
CD3CN (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4))
4))
| Protons | δ/ppm ligand 1 | δ/ppm [Zn2(1)]4+ | δ/ppm [Cu2(1)]2+ |
|---|---|---|---|
| a-H | 8.57 | 6.56 | 6.67 |
| b-H | 7.44 | 7.01 | 7.10 |
| 4-H | 8.49 | 8.94 | 8.77 |
| 5 and 6-H | 7.91, 7.80 | 8.27, 8.17 | 8.13, 8.06 |
| 7-H | 8.32 | 8.87 | 8.66 |
| 8-H | 7.57 | 7.97 | 7.90 |
| 9 and 9′-H | 6.97, 6.96 | 6.94 | 6.96 |
The stochastic rotational exchange in nanorotors [Zn2(1)]4+ and [Cu2(1)]2+ occurs at activation barriers 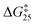 of 50.5 and 47.4 kJ mol−1, respectively. Since, departure of the rotator arm is endergonic and rate determining3a it was expected that rotor [Cu2(1)]2+ has a slightly higher rotational rate than [Zn2(1)]4+ because the pyridine → copper(I) (18.3 kJ mol−1)3e is less endergonic than the pyridine → zinc(II) dissociation (22.2 kJ mol−1; Fig. S65†). Moreover, dissociation of pyridine from the metal center may be accelerated via an SN2 displacement by nucleophilic components in the solvent, e.g. acetonitrile.12
of 50.5 and 47.4 kJ mol−1, respectively. Since, departure of the rotator arm is endergonic and rate determining3a it was expected that rotor [Cu2(1)]2+ has a slightly higher rotational rate than [Zn2(1)]4+ because the pyridine → copper(I) (18.3 kJ mol−1)3e is less endergonic than the pyridine → zinc(II) dissociation (22.2 kJ mol−1; Fig. S65†). Moreover, dissociation of pyridine from the metal center may be accelerated via an SN2 displacement by nucleophilic components in the solvent, e.g. acetonitrile.12
To probe the interdependent system of rotor and fluorescence reporter, ligands 1 and 2 were mixed (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
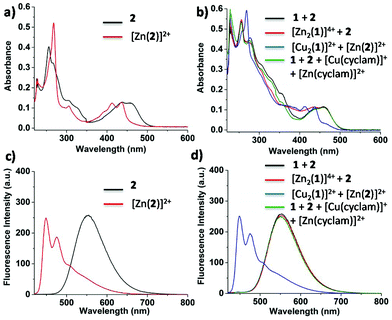
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The UV-vis analysis in 0.2% CH3CN in CH2Cl2 displayed absorptions at λ = 255, 438 and 458 nm, characteristic of receptor 2 (Fig. 4a). After addition of two equiv. of Zn(OTf)2, nanorotor [Zn2(1)]4+ was afforded while receptor 2 remained unloaded, as suggested in NMR (Fig. 2b) and also by UV-vis and emission spectra. The UV-vis spectrum still displayed the absorption bands of the free receptor 2 at λ = 255, 438 and 458 nm (Fig. 4a and b). Equally, the emission profile did not change after addition of zinc(II); the maximum remained at λ = 552 nm as in the case of receptor 2 only (Fig. 4c and d). It is important to note that the zinc(II) rotor does not exhibit any emission when excited at λ = 410 nm.
2). The UV-vis analysis in 0.2% CH3CN in CH2Cl2 displayed absorptions at λ = 255, 438 and 458 nm, characteristic of receptor 2 (Fig. 4a). After addition of two equiv. of Zn(OTf)2, nanorotor [Zn2(1)]4+ was afforded while receptor 2 remained unloaded, as suggested in NMR (Fig. 2b) and also by UV-vis and emission spectra. The UV-vis spectrum still displayed the absorption bands of the free receptor 2 at λ = 255, 438 and 458 nm (Fig. 4a and b). Equally, the emission profile did not change after addition of zinc(II); the maximum remained at λ = 552 nm as in the case of receptor 2 only (Fig. 4c and d). It is important to note that the zinc(II) rotor does not exhibit any emission when excited at λ = 410 nm.
To test the communication via metal translocation, two equiv. of [Cu(CH3CN)]PF6 were added. 1H NMR results suggested that 91% of the copper(I) rotor [Cu2(1)]2+ formed while parallel Zn2+ was translocated to receptor 2 (Fig. 2c). The NMR signals of 2 changed on the way to [Zn(2)]2+, e.g. proton k-H shifted downfield from 6.71 to 6.87 ppm. At the same time, the UV-vis analysis equally validated that Zn2+ had translocated to receptor 2 to afford [Zn(2)]2+ (Fig. 4b), because absorption bands appeared at λ = 268, 412 and 438 nm, identical to the absorptions of separately prepared [Zn(2)]2+. Parallel, emission spectroscopy exhibited a blue-shifted fluorescence at λ = 448 and 475 nm (Fig. 4d). Finally, four equivalents of cyclam were added to capture all copper(I) and zinc(II) ions and to resume the initial state (Fig. 2d). The process of sequential addition of metal ions (Zn2+ and Cu+) and of cyclam was repeated up to two cycles (ESI) proving full reversibility of the networking by 1H NMR and emission data.
Upon addition of two equiv. of [Cu(CH3CN)4]PF6 with respect to rotor [Zn2(1)]4+ (3.60 μM) in the presence of receptor 2 (7.20 μM), translocation of Zn2+ from ligand 1 to receptor 2 took approximately 60 min in 0.2% CH3CN in CH2Cl2 by UV-Vis. Due to an intermittent absorbance at 268 nm, attributed to [Cu(2)]+, the mechanism is suggested as follows: first, the copper(I) ion coordinates to aza-crown 2, then the copper(I) and zinc(II) ions slowly exchange their binding sites due to global thermodynamics since the coordination of Cu+ to the phenanthroline ligand (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 5.42) is stronger than that of Zn2+ (log
K = 5.42) is stronger than that of Zn2+ (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 4.58) while both ions have almost identical binding data to 2 (log
K = 4.58) while both ions have almost identical binding data to 2 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 4.08 vs. 3.91). Kinetic analysis suggests a first-order kinetics (t1/2 = 762 s at 25 °C) for translocation.
K = 4.08 vs. 3.91). Kinetic analysis suggests a first-order kinetics (t1/2 = 762 s at 25 °C) for translocation.
The nicely agreeing emission signals of the black and green emission traces in Fig. 4d demonstrate the full reversibility of the system when it is reset by addition of cyclam.
Conclusion
In conclusion, we prepared the covalent turnstile 1 with two phenanthroline stations in the stator part and a pyridine arm as rotator, whose rotational barrier at room temperature is estimated as 2.4–2.7 kJ mol−1.9 Nanorotor [Zn2(1)]4+, formed upon addition of Zn2+, operates at k25 = 8000 Hz and the Cu+-loaded system [Cu2(1)]2+ rotates at k25 = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz. The three-step transformation of 1 (>109 kHz) → [Zn2(1)]4+ (8 kHz) → [Cu2(1)]2+ (30 kHz) was achieved by the sequential addition of zinc(II) and copper(I) ions with the last transformation being monitored by 2 as fluorescence reporter. While the ensemble of rotor [Zn2(1)]4+ and free receptor 2 exhibited an emission at 552 nm, the addition of copper(I) triggering zinc(II) translocation (t1/2 = 762 s) to afford receptor [Zn(2)]2+ shifted the emission to λ = 448 and 475 nm. Fully reversible ion exchange between 1 and receptor 2 was demonstrated over two cycles, indicating that multifunctional (i.e. rotation & emission) and multicomponent systems work in a reliable and coherent manner.
000 Hz. The three-step transformation of 1 (>109 kHz) → [Zn2(1)]4+ (8 kHz) → [Cu2(1)]2+ (30 kHz) was achieved by the sequential addition of zinc(II) and copper(I) ions with the last transformation being monitored by 2 as fluorescence reporter. While the ensemble of rotor [Zn2(1)]4+ and free receptor 2 exhibited an emission at 552 nm, the addition of copper(I) triggering zinc(II) translocation (t1/2 = 762 s) to afford receptor [Zn(2)]2+ shifted the emission to λ = 448 and 475 nm. Fully reversible ion exchange between 1 and receptor 2 was demonstrated over two cycles, indicating that multifunctional (i.e. rotation & emission) and multicomponent systems work in a reliable and coherent manner.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge generous financial support from the Deutsche Forschungsgemeinschaft (DFG Schm 647/20-2).Notes and references
- (a) J. M. Abendroth, O. S. Bushuyev, P. S. Weiss and C. J. Barrett, ACS Nano, 2015, 9, 7746–7768 CrossRef CAS PubMed; (b) S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Chem. Rev., 2015, 115, 10081–10206 CrossRef CAS PubMed.
- (a) B. Godde, D. Ritaine, A. Jouaiti, M. Mauro and M. W. Hosseini, New J. Chem., 2018, 42, 7810–7815 RSC; (b) B. Godde, A. Jouaiti, A. Fluck, N. Kyritsakas, M. Mauro and M. W. Hosseini, Dalton Trans., 2017, 46, 14897–14906 RSC; (c) G. Wang, H. Xiao, J. He, J. Xiang, Y. Wang, X. Chen, Y. Che and H. Jiang, J. Org. Chem., 2016, 81, 3364–3371 CrossRef CAS PubMed; (d) Z. Zhou, X. Zhang, Q. Liu, Z. Yan, C. Lv and G. Long, Inorg. Chem., 2013, 52, 10258–10263 CrossRef CAS PubMed.
- (a) P. K. Biswas, S. Saha, Y. Nanaji, A. Rana and M. Schmittel, Inorg. Chem., 2017, 56, 6662–6670 CrossRef CAS; (b) A. Goswami, I. Paul and M. Schmittel, Chem. Commun., 2017, 53, 5186–5189 RSC; (c) M. Nakamura, K. Kishimoto, Y. Kobori, T. Abe, K. Yoza and K. Kobayashi, J. Am. Chem. Soc., 2016, 138, 12564–12577 CrossRef CAS PubMed; (d) M. Krick, J. Holstein, C. Würtele and G. H. Clever, Chem. Commun., 2016, 52, 10411–10414 RSC; (e) S. K. Samanta and M. Schmittel, J. Am. Chem. Soc., 2013, 135, 18794–18797 CrossRef CAS PubMed; (f) B. E. Dial, P. J. Pellechia, M. D. Smith and K. D. Shimizu, J. Am. Chem. Soc., 2012, 134, 3675–3678 CrossRef CAS PubMed; (g) S. Hiraoka, Y. Hisanaga, M. Shiro and M. Shionoya, Angew. Chem., Int. Ed., 2010, 49, 1669–1673 CrossRef CAS PubMed.
- J. Michl and E. C. H. Sykes, ACS Nano, 2009, 3, 1042–1048 CrossRef CAS PubMed.
- P. K. Biswas, S. Saha, T. Paululat and M. Schmittel, J. Am. Chem. Soc., 2018, 140, 9038–9041 CrossRef CAS PubMed.
- (a) Y. Wu, G. Wang, Q. Li, J. Xiang, H. Jiang and Y. Wang, Nat. Commun., 2018, 9, 1953 CrossRef PubMed; (b) G. T. Rushton, E. C. Vik, W. G. Burns, R. D. Rasberry and K. D. Shimizu, Chem. Commun., 2017, 53, 12469–12472 RSC; (c) A. Faulkner, T. van Leeuwen, B. L. Feringa and S. J. Wezenberg, J. Am. Chem. Soc., 2016, 138, 13597–13603 CrossRef CAS; (d) Y. Suzuki, M. Kageyama, R. Morisawa, Y. Dobashi, H. Hasegawa, S. Yokojima and O. Kitagawa, Chem. Commun., 2015, 51, 11229–11232 RSC.
- N. Zigon, P. Larpent, A. Jouaiti, N. Kyritsakas and M. W. Hosseini, Dalton Trans., 2014, 43, 15779–15784 RSC.
- (a) M. Schmittel, Dalton Trans., 2018, 47, 6654–6659 RSC; (b) A. Goswami, S. Pramanik and M. Schmittel, Chem. Commun., 2018, 54, 3955–3958 RSC; (c) N. Mittal, S. Pramanik, I. Paul, S. De and M. Schmittel, J. Am. Chem. Soc., 2017, 139, 4270–4273 CrossRef CAS PubMed.
- (a) N. M. Albu, E. Bergin and D. J. Yaron, J. Phys. Chem. A, 2009, 113, 7090–7096 CrossRef CAS PubMed; (b) S. J. Greaves, E. L. Flynn, E. L. Futcher, E. Wrede, D. P. Lydon, P. J. Low, S. R. Rutter and A. Beeby, J. Phys. Chem. A, 2006, 110, 2114–2121 CrossRef CAS PubMed.
- (a) M. Schmittel, B. He, J. Fan, J. W. Bats, M. Engeser, M. Schlosser and H.-J. Deiseroth, Inorg. Chem., 2009, 48, 8192–8200 CrossRef CAS PubMed; (b) S. Neogi, G. Schnakenburg, Y. Lorenz, M. Engeser and M. Schmittel, Inorg. Chem., 2012, 51, 10832–10841 CrossRef CAS PubMed.
- M. L. Saha and M. Schmittel, Org. Biomol. Chem., 2012, 10, 4651–4684 RSC.
- S. Saha, P. K. Biswas and M. Schmittel, Inorg. Chem., 2019, 58, 3466–3472 CrossRef CAS PubMed.
- H. J. Reich, NMR Spectrum Calculations: WinDNMR, Version 7.1, Department of Chemistry, University of Wisconsin Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, compound characterizations, spectral data, UV-vis titrations data. See DOI: 10.1039/c9dt01633c |
| This journal is © The Royal Society of Chemistry 2019 |