Synthesis and structural, redox and photophysical properties of tris-(2,5-di(2-pyridyl)pyrrolide) lanthanide complexes†
Abstract
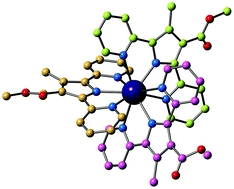
A first series of lanthanide complexes of tris(dipyridyl)pyrrolide ligands has been prepared. The [Ln(dppR1,R2)3] complexes (Ln = La(III), Sm(III), Eu(III), Gd(III) and Yb(III); and dppR1,R2 = 2,5-di(2-pyridyl-3-(R1)-4-(R2)) pyrrolide) have been isolated and their structures and photophysical and redox properties characterised, both in the solid-state and in solution. In the complexes, the three dpp− ligands form a distorted tricapped trigonal prismatic coordination geometry about the lanthanide ions, with the antiparallel isomer observed in the solid state for non-symmetric (dppCO2Me,Me)−. However, 1H NMR spectroscopy of the diamagnetic and paramagnetic [Ln(dppR1,R2)3] complexes in d6-benzene solution reveal evidence for a statistical distribution of all possible isomers. Time-resolved luminescence studies suggest that the dpp− ligand (with triplet excited state T1 energy at 18 622 cm−1) sensitises red emission from [Eu(dppCO2Me,Me)3] and near-infrared emission from [Yb(dppCO2Me,Me)3] through the antenna effect. Cyclic voltammetry reveals three consecutive, reversible, one-electron oxidation processes for each [Ln(dppR1,R2)3] complex, corresponding to oxidations of each dpp− ligand between 0.3–0.8 V vs. E1/2 (Fc+/0), and for [Eu(dppCO2Me,Me)3] the EuIII/II couple was −2.099 V vs. E1/2 (Fc+/0).


 Please wait while we load your content...
Please wait while we load your content...