Inducing selectivity and chirality in group IV metal coordination with high-denticity hydroxypyridinones†
Abstract
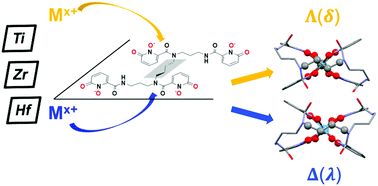
The solution- and solid-state interactions between the octadentate siderophore mimic 3,4,3-LI(1,2-HOPO) (343HOPO) and group IV metal ions were investigated using high-resolution mass spectrometry, liquid chromatography, UV-visible spectrophotometry, metal-competition batch titrations, and single crystal X-ray diffraction. 343HOPO forms a neutral 1 : 1 complex, [HfIV343HOPO], that exhibits extreme stability in aqueous solution, with a log β110 value reaching 42.3. These results affirm the remarkable charge-based selectivity of 343HOPO for octacoordinated tetravalent cations with a Hf(IV) complex 1021 more stable than its Lu(III) analogue. Moreover, [HfIV343HOPO] and its Zr(IV) counterpart show exceptional robustness, with the ligand remaining bound to the cation over a very broad pH range: from pH ∼ 11 to acidic conditions as strong as 10 M HCl. In stark contrast, Ti(IV)-343HOPO species are far less stable and undergo hydrolysis at pH as low as ∼6, likely due to the mismatch between the preferred hexacoordinated Ti(IV) ion and octadentate 343HOPO ligand. The extreme charge-based and denticity-driven selectivity of 343HOPO, now observed across the periodic table, paves the way for new selective sequestration systems for radionuclides including medical 44Ti, 89Zr or 177Lu/Hf isotopes, toxic polonium (Po) contaminants, as well as rutherfordium (Rf) research isotopes. Furthermore, despite the lack of a chiral center in 343HOPO, its complexes with metal ions are chiral and appear to form a single set of enantiomers.


 Please wait while we load your content...
Please wait while we load your content...