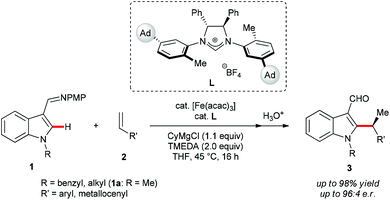
Open Access Article
Open Access ArticleMössbauer and mass spectrometry support for iron(II) catalysts in enantioselective C–H activation†
Joachim
Loup
 a,
Tobias
Parchomyk
a,
Stefan
Lülf
a,
Serhiy
Demeshko
b,
Franc
Meyer
a,
Tobias
Parchomyk
a,
Stefan
Lülf
a,
Serhiy
Demeshko
b,
Franc
Meyer
 b,
Konrad
Koszinowski
b,
Konrad
Koszinowski
 *a and
Lutz
Ackermann
*a and
Lutz
Ackermann
 *a
*a
aInstitut für Organische und Biomolekulare Chemie, Universität Göttingen, Tammannstraße 2, 37077 Göttingen, Germany. E-mail: Konrad.Koszinowski@chemie.uni-goettingen.de; Lutz.Ackermann@chemie.uni-goettingen.de
bInstitut für Anorganische Chemie, Universität Göttingen, Tammannstraße 4, 37077 Göttingen, Germany
First published on 21st March 2019
Abstract
A combination of electrospray-ionization mass spectrometry and Mössbauer spectroscopy was used to investigate the species generated in situ in highly enantioselective Fe/NHC-catalyzed C–H alkylations. The findings indicate an organometallic iron(II)–NHC species to be of key relevance in the asymmetric catalysis.
Iron has emerged as a sustainable, inexpensive and non-toxic alternative to noble transition metals for catalysis,1 and applications to cross-coupling chemistry2 and C–H activation3 have experienced a considerable development in recent years. Notably, the association of N-heterocyclic carbene (NHC) ligands with iron is of particular interest and has been exploited for C–C and C–X forming processes.4
Within our program on iron-catalyzed C–H activation,5 we have very recently developed the first example of enantioselective iron-catalyzed C–H functionalizations by inner-sphere organometallic C–H activation.6 Key to success was the design of the novel NHC ligand L featuring remote meta-adamantyl substituents (Scheme 1). While detailed kinetic studies and deuterium labelling experiments were performed to delineate the modus operandi of the transformation, the oxidation state and coordination sphere of the active catalyst remained thus far elusive.
Indeed, in iron/NHC catalyzed C–H activations,6,7 as well as in related low-valent cobalt/NHC-catalyzed C–H activations,8 the active catalyst is generated in situ from a metal salt and a imidazol(in)ium NHC precursor in the presence of a Grignard reagent. The organometallic reagent is assumed to play a dual role serving both as base and reductant, but no well-defined complex has been so far isolated or characterized in this context, with the notable exception of Tatsumi/Ohki employing a half sandwich Fe(II)/NHC complex for undirected C–H borylations.9
Based on preliminary mechanistic studies and previous reports,10 we initially proposed an organometallic Fe/NHC species to be the active catalyst and the key C–H cleavage event to occur via a ligand-to-ligand hydrogen-transfer (LLHT) manifold.11 On the contrary, Yoshikai originally proposed a low-valent Fe/NHC complex with an oxidation state <+2 generated through the reduction of the iron(III) pre-catalyst by the Grignard reagent to be operative in a racemic hydroarylation of vinylarenes and alkynes with indoles, and the C–H activation step to occur via C–H oxidative addition.7 It is noteworthy that Yoshikai attributed the requirement of an excess of the Grignard reagent to the possible formation of ferrate species.
In the course of our earlier studies, we observed that Fe(acac)3 and FeCl2 pre-catalysts, despite their different oxidation states and counterions, gave comparable conversions and enantio-selectivities. This observation was suggestive of the iron precursors being transformed by the Grignard reagent to the same catalytically competent iron species. Another possibility is the in situ formation of organoferrates, in agreement with recent reports that the nature of the iron precursor has very little effect on the transmetallation reactions with organometallic species to form such complexes.12 These observations raised the question as to the nature of the active catalyst and its mode of action, and highlight the need for detailed, comprehensive mechanistic studies to unravel fundamental aspects of iron-catalyzed C–H activations. Such mechanistic insights have recently been gained for iron-catalyzed Kumada–Corriu-type cross-coupling reactions via Mössbauer spectroscopy and mass spectrometry.12,13 These reports highlighted the dynamic nature and remarkable complexity of organometallic iron chemistry.
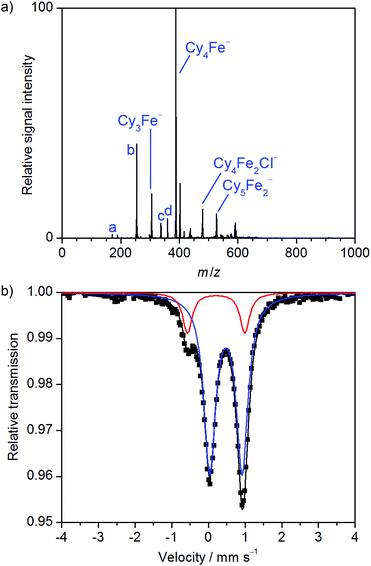
Hence, we became interested in the application of electrospray-ionization (ESI) mass spectrometry and Mössbauer spectroscopy to elucidate the key intermediates formed in situ in the enantioselective iron-catalyzed C–H alkylation. Mössbauer spectroscopy has the advantage of probing the entire population of iron species, regardless of their individual charge. We decided to follow a step-by-step approach and therefore initiated our investigation by probing the species formed in the reaction between the iron pre-catalyst, the Grignard reagent and N,N,N′,N′-tetramethylethane-1,2-diamine (TMEDA) in THF, without the NHC precursor or the indole substrate. Negative-ion mode ESI mass spectra of a solution of Fe(acac)3 treated with 8.0 equiv. of CyMgCl in the presence of TMEDA (4.0 equiv.) showed a mixture of various organoferrate species, among which Cy3Fe(II)− and Cy4Fe(III)− predominated (Fig. 1a). Previous work had already established the formation of abundant organoferrate anions upon transmetallation of iron precursors with Grignard reagents.12 Although ESI mass spectrometry cannot directly detect any neutral species, the observation of small amounts of the anions Cy5Fe2− and Cy4Fe2Cl− – both with iron in an average oxidation state of II – indicated the presence of neutral organoiron complexes, such as Cy2Fe or CyFeCl, which supposedly reacted with Cy3Fe(II)− to afford the dinuclear aggregates. The low abundance of the dinuclear anions can be ascribed to the addition of TMEDA, which had previously been shown to prevent the formation of polynuclear organoferrates.10a,12,14 All of the detected organoferrates were found to be highly unstable, presumably due to β-hydride elimination, and completely disappeared within minutes. Experiments conducted with the more stable and user-friendly PhMgCl were hence performed as well. PhMgCl had previously been shown to effect the desired C–H alkylation as well, albeit with a slightly diminished performance.6 Likewise, iron(II) and iron(III) phenylferrates were observed by ESI-MS in the reaction of Fe(acac)3 with PhMgCl in the presence of TMEDA (Fig. S1†), being in full agreement with previous findings.10a,12,15
We next analyzed a frozen solution of 57FeCl2/CyMgCl/TMEDA by 57Fe Mössbauer spectroscopy at 80 K (Fig. 1b). The obtained spectrum featured the signatures of two iron species, which were assigned to a major high-spin iron(III) species and a minor low-coordinate iron(II) species, in line with the formation of Cy4Fe(III)− and Cy3Fe(II)− observed by ESI-MS. The remarkable formation of a dominating iron(III) species from the iron(II) precursor in the presence of Grignard reagents and the absence of any external oxidant is attributed to disproportionation with concomitant formation of a low-valent iron species.12,16 Interestingly, we did not detect any Cy4Fe(IV), which had been observed in a related setting.19 The instability of the cyclohexylferrates was further highlighted by 57Fe Mössbauer spectroscopic analysis of the same reaction after it was allowed to warm to 23 °C (Fig. S3†). The spectrum showed the complete disappearance of the iron(II) ate complex, a reduced amount of Cy4Fe(III)− and the emergence of a new dominant species, whose unspecific doublet unfortunately does not allow for assignment.
A similar spectrum was obtained from the reaction of 57FeCl2 with PhMgCl in the presence of TMEDA (Fig. S4†), indicating the formation of the phenylferrates Ph3Fe(II)− and Ph4Fe(III)−, being in line with the ESI-MS results and previous reports.10a,12 The parameters of the Mössbauer doublet assigned to Ph3Fe(II)− (δ = 0.20 mm s−1, ΔEQ(red) = 1.44 mm s−1) are indeed very similar to those reported for the closely related Mes3Fe(II)− (δ = 0.21 mm s−1, ΔEQ = 1.43 mm s−1).13h The dimeric dianion [{Ph2Fe(μ-Ph)}2]2− (δ = 0.34 mm s−1 and ΔEQ = 2.28 mm s−1 at 80 K), previously obtained from the low temperature reaction of Fe(acac)3 and PhMgBr in THF, has a higher isomer shift and much larger quadrupole splitting, and the more reduced neutral tetramer Fe4(μ-Ph)6(solv)x (δ = 0.60 mm s−1 and ΔEQ = 0.84 mm s−1 at 80 K) has a significantly higher isomer shift.13j As the catalyzed C–H activation was found to be completely shut down in the absence of the ligand,6 the observed ligand-free organoferrates are assumed to be catalytically inactive.
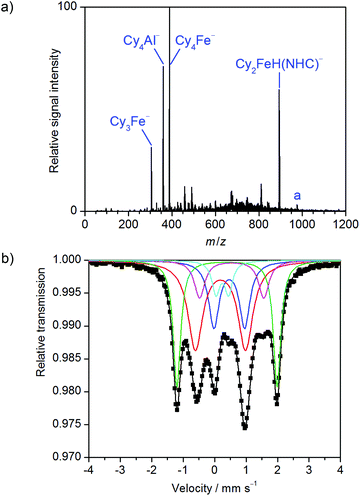
Subsequently, similar experiments were performed in the presence of the chiral NHC precursor L (Fig. 2a). While the homoleptic ferrates remained present in the solution, two newly formed iron(II) species could also be observed, namely Cy3Fe(NHC)− and Cy2FeH(NHC)− (Fig. S5 and S6†). The latter, with a significantly higher intensity, is believed to result from β-hydrogen elimination of the former. Interestingly, no NHC complexes of iron(III) or low-valent iron were detected, suggesting the selective formation of Fe(II)/NHC species in the reaction.
When a similar experiment was performed using PhMgCl, no Fe/NHC species could be observed (Fig. S7†). Yet, the relative intensity of the iron(II) ate complex was noticeably reduced, which presumably indicates its consumption to form neutral species not detectable by ESI mass spectrometry. Besides, no magnesium-NHC complexes or residual imidazolinium salt could be observed by positive-mode ESI-MS in any of the experiments (Fig. S8†), providing evidence for the NHC to coordinate to the iron catalyst, and not to Mg(II).
When a frozen solution of 57FeCl2/L/CyMgCl/TMEDA was analyzed by Mössbauer spectroscopy, a rather complicated spectrum could be observed which has been simulated well assuming five subspectra.20 Two subspectra (Fig. 2b, blue and red) are almost identical to the previously observed ferrates (Fig. 1b). The most intense new signal (Fig. 2b, green, 27% intensity) with a large quadrupole splitting of 3.19 mm s−1 but with quite a low isomer shift of 0.39 mm s−1 can be assigned to a low-coordinate iron(II) high-spin complex, most likely trigonal-planar Cy2Fe(NHC),21 in line with ESI mass spectrometry (see above). It should be noted that the isomer shifts of L2Fe(NHC) complexes can vary depending on the covalency of the Fe–C bond. Examples include δ = 0.33 to 0.35 mm s−1 at 80 K for [(Me3Si)CH2]2Fe(NHC),21cδ = 0.33 mm s−1 at 200 K for (Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh)2Fe(NHC),21d or δ = 0.44 to 0.57 mm s−1 at 5 K for (1,3-dioxan-2-ylethyl)2Fe(NHC);21a all these complexes were shown to adopt a high-spin Fe(II) state. Another newly formed species (magenta), with a higher isomer shift of 0.54 mm s−1 together with a lower quadrupole splitting of 2.04 mm s−1, may indicate a more symmetric iron(II) high-spin species with a higher coordination number such as Cy3Fe(NHC)−, as observed by ESI-MS (see Fig. S5†). An additional minor species (Fig. 2b, cyan) was also observed in the 57Fe Mössbauer spectrum of the reaction with CyMgCl, but its non-characteristic doublet does not allow for further assignment. Two similar iron(II) species were also detected in the analogous reaction with PhMgCl (Fig. S9†). No species related to the minor uncharacteristic signal observed previously was however detected in this system. It is hence believed that this species was formed via β-hydride elimination from the Cy2Fe(NHC) complex. This hypothesis is substantiated by the observation that, when the sample was prepared at higher temperatures, this species (cyan) became more pronounced, while the intensity of the Cy2Fe(NHC) signal (green) was reduced (Fig. S10†).
CPh)2Fe(NHC),21d or δ = 0.44 to 0.57 mm s−1 at 5 K for (1,3-dioxan-2-ylethyl)2Fe(NHC);21a all these complexes were shown to adopt a high-spin Fe(II) state. Another newly formed species (magenta), with a higher isomer shift of 0.54 mm s−1 together with a lower quadrupole splitting of 2.04 mm s−1, may indicate a more symmetric iron(II) high-spin species with a higher coordination number such as Cy3Fe(NHC)−, as observed by ESI-MS (see Fig. S5†). An additional minor species (Fig. 2b, cyan) was also observed in the 57Fe Mössbauer spectrum of the reaction with CyMgCl, but its non-characteristic doublet does not allow for further assignment. Two similar iron(II) species were also detected in the analogous reaction with PhMgCl (Fig. S9†). No species related to the minor uncharacteristic signal observed previously was however detected in this system. It is hence believed that this species was formed via β-hydride elimination from the Cy2Fe(NHC) complex. This hypothesis is substantiated by the observation that, when the sample was prepared at higher temperatures, this species (cyan) became more pronounced, while the intensity of the Cy2Fe(NHC) signal (green) was reduced (Fig. S10†).
Thereafter, additional experiments in the presence of the indole substrate 1a were conducted. Besides the previously observed species, [Cy4Fe(indole)]− was observed by ESI-MS analysis of the reaction of 57FeCl2/CyMgCl/TMEDA/L/1a (Fig. S11†). Yet, this species is believed to be catalytically irrelevant in the C–H activation due to the absence of the NHC ligand. ESI-MS analysis of the reaction of 57FeCl2/PhMgCl/TMEDA/L/1a showed no new species, but the almost complete disappearance of the Ph3Fe(II)− ferrate (Fig. S12†). Its consumption is suggestive of a reaction between the iron(II) ate complex, or a species in equilibrium with it, and the substrate 1a to form a neutral species. Therefore, this observation is suggestive of an iron(II) species to be involved as intermediate in the C–H activation.
No new species or significant changes upon the addition of substrate 1a were observed by 57Fe Mössbauer spectroscopy analysis of the analogous reactions with either CyMgCl or PhMgCl, except a slight reduction of the species believed to be R2Fe(NHC) (Fig. S13 and S14†).
In summary, we report on the unprecedented application of ESI-MS and 57Fe Mössbauer spectroscopy to study the mechanism of iron-catalyzed asymmetric C–H activations. Our experimental findings provide support for the formation of an organometallic Fe(II)/NHC complex as an intermediate in the iron-catalyzed enantioselective C–H alkylation of indoles. Furthermore, no interaction between iron and TMEDA was observed in any of the experiments, which suggests that TMEDA coordinates the Mg(II) ions and does not interact with the iron catalyst.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Generous support by the DFG (SPP 1807, KO 2875/10-1, KO 2875/8-1, AC 118/7-1 and Gottfried-Wilhelm-Leibniz award) and the Georg-August University Göttingen is gratefully acknowledged.References
- (a) A. Fürstner, ACS Cent. Sci., 2016, 2, 778 CrossRef PubMed; (b) I. Bauer and H.-J. Knölker, Chem. Rev., 2015, 115, 3170 CrossRef CAS PubMed; (c) C. Bolm, J. Legros, J. Le Paih and L. Zani, Chem. Rev., 2004, 104, 6217 CrossRef CAS PubMed.
- (a) A. Piontek, E. Bisz and M. Szostak, Angew. Chem., Int. Ed., 2018, 57, 11116 CrossRef CAS PubMed; (b) T. L. Mako and J. A. Byers, Inorg. Chem. Front., 2016, 3, 766 RSC; (c) C. Cassani, G. Bergonzini and C.-J. Wallentin, ACS Catal., 2016, 6, 1640 CrossRef CAS; (d) E. Nakamura, T. Hatakeyama, S. Ito, K. Ishizuka, L. Ilies and M. Nakamura, Org. React., 2014, 83, 1 CAS; (e) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417 CrossRef CAS PubMed; (f) E. Nakamura and N. Yoshikai, J. Org. Chem., 2010, 75, 6061 CrossRef CAS; (g) W. M. Czaplik, M. Mayer, J. Cvengroš and A. J. von Wangelin, ChemSusChem, 2009, 2, 396 CrossRef CAS PubMed; (h) B. D. Sherry and A. Fürstner, Acc. Chem. Res., 2008, 41, 1500 CrossRef CAS PubMed.
- (a) N. Yoshikai, Isr. J. Chem., 2017, 57, 1117 CrossRef CAS; (b) R. Shang, L. Ilies and E. Nakamura, Chem. Rev., 2017, 117, 9086 CrossRef CAS PubMed; (c) G. Cera and L. Ackermann, Top. Curr. Chem., 2016, 374, 57 CrossRef.
- (a) C. Johnson and M. Albrecht, Coord. Chem. Rev., 2017, 352, 1 CrossRef CAS; (b) K. Riener, S. Haslinger, A. Raba, M. P. Högerl, M. Cokoja, W. A. Herrmann and F. E. Kühn, Chem. Rev., 2014, 114, 5215 CrossRef CAS PubMed; (c) D. Bézier, J.-B. Sortais and C. Darcel, Adv. Synth. Catal., 2013, 355, 19 CrossRef.
- Selected examples: (a) J. Mo, T. Müller, J. C. A. Oliveira and L. Ackermann, Angew. Chem., Int. Ed., 2018, 57, 7719 CrossRef CAS; (b) Z. Shen, G. Cera, T. Haven and L. Ackermann, Org. Lett., 2017, 19, 3795 CrossRef CAS PubMed; (c) G. Cera, T. Haven and L. Ackermann, Chem. Commun., 2017, 53, 6460 RSC; (d) G. Cera, T. Haven and L. Ackermann, Chem. – Eur. J., 2017, 23, 3577 CrossRef CAS; (e) G. Cera, T. Haven and L. Ackermann, Angew. Chem., Int. Ed., 2016, 55, 1484 CrossRef CAS PubMed; (f) K. Graczyk, T. Haven and L. Ackermann, Chem. – Eur. J., 2015, 21, 8812 CrossRef CAS; (g) Q. Gu, H. H. Al Mamari, K. Graczyk, E. Diers and L. Ackermann, Angew. Chem., Int. Ed., 2014, 53, 3868 CrossRef CAS PubMed.
- J. Loup, D. Zell, J. C. A. Oliveira, H. Keil, D. Stalke and L. Ackermann, Angew. Chem., Int. Ed., 2017, 56, 14197 CrossRef CAS PubMed.
- M. Y. Wong, T. Yamakawa and N. Yoshikai, Org. Lett., 2015, 17, 442 CrossRef CAS.
- (a) N. Sauermann, J. Loup, D. Kootz, V. R. Yatham, A. Berkessel and L. Ackermann, Synthesis, 2017, 49, 3476 CrossRef CAS; (b) M. Moselage, N. Sauermann, S. C. Richter and L. Ackermann, Angew. Chem., Int. Ed., 2015, 54, 6352 CrossRef CAS; (c) J. Li and L. Ackermann, Chem. – Eur. J., 2015, 21, 5718 CrossRef CAS PubMed; (d) B. Punji, W. Song, G. A. Shevchenko and L. Ackermann, Chem. – Eur. J., 2013, 19, 10605 CrossRef CAS; (e) K. Gao and N. Yoshikai, J. Am. Chem. Soc., 2013, 135, 9279 CrossRef CAS PubMed; (f) Z. Ding and N. Yoshikai, Angew. Chem., Int. Ed., 2013, 52, 8574 CrossRef CAS PubMed; (g) W. Song and L. Ackermann, Angew. Chem., Int. Ed., 2012, 51, 8251 CrossRef CAS; (h) K. Gao and N. Yoshikai, Chem. Commun., 2012, 48, 4305 RSC. Selected reviews: (i) M. Moselage, J. Li and L. Ackermann, ACS Catal., 2016, 6, 498 CrossRef CAS; (j) K. Gao and N. Yoshikai, Acc. Chem. Res., 2014, 47, 1208 CrossRef CAS PubMed; (k) L. Ackermann, J. Org. Chem., 2014, 79, 8948 CrossRef CAS.
- (a) T. Hatanaka, Y. Ohki and K. Tatsumi, Chem. – Asian J., 2010, 5, 1657 CrossRef CAS PubMed; (b) Y. Ohki, T. Hatanaka and K. Tatsumi, J. Am. Chem. Soc., 2008, 130, 17174 CrossRef CAS PubMed.
- (a) T. Parchomyk and K. Koszinowski, Chem. – Eur. J., 2016, 22, 15609 CrossRef CAS PubMed; (b) R. B. Bedford, P. B. Brenner, D. Elorriaga, J. N. Harvey and J. Nunn, Dalton Trans., 2016, 45, 15811 RSC; (c) R. B. Bedford, P. B. Brenner, E. Carter, P. M. Cogswell, M. F. Haddow, J. N. Harvey, D. M. Murphy, J. Nunn and C. H. Woodall, Angew. Chem., Int. Ed., 2014, 53, 1804 CrossRef CAS; (d) M. D. Greenhalgh and S. P. Thomas, J. Am. Chem. Soc., 2012, 134, 11900 CrossRef CAS; (e) E. Shirakawa, D. Ikeda, S. Masui, M. Yoshida and T. Hayashi, J. Am. Chem. Soc., 2012, 134, 272 CrossRef CAS.
- (a) D. Zell, M. Bursch, V. Müller, S. Grimme and L. Ackermann, Angew. Chem., Int. Ed., 2017, 56, 10378 CrossRef CAS PubMed; (b) O. Eisenstein, J. Milani and R. N. Perutz, Chem. Rev., 2017, 117, 8710 CrossRef CAS PubMed; (c) J. Guihaumé, S. Halbert, O. Eisenstein and R. N. Perutz, Organometallics, 2012, 31, 1300 CrossRef; (d) L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef CAS PubMed.
- T. Parchomyk, S. Demeshko, F. Meyer and K. Koszinowski, J. Am. Chem. Soc., 2018, 140, 9709 CrossRef CAS PubMed.
- (a) J. D. Sears, P. G. N. Neate and M. L. Neidig, J. Am. Chem. Soc., 2018, 140, 11872 CrossRef CAS PubMed; (b) T. Parchomyk and K. Koszinowski, Synthesis, 2017, 49, 3269 CrossRef CAS; (c) R. B. Bedford, Acc. Chem. Res., 2015, 48, 1485 CrossRef CAS PubMed; (d) S. H. Carpenter and M. L. Neidig, Isr. J. Chem., 2017, 57, 1106 CrossRef CAS PubMed; (e) S. B. Muñoz III, S. L. Daifuku, J. D. Sears, T. M. Baker, S. H. Carpenter, W. W. Brennessel and M. L. Neidig, Angew. Chem., Int. Ed., 2018, 57, 6496 CrossRef PubMed; (f) J. L. Kneebone, W. W. Brennessel and M. L. Neidig, J. Am. Chem. Soc., 2017, 139, 6988 CrossRef CAS PubMed; (g) S. B. Muñoz III, S. L. Daifuku, W. W. Brennessel and M. L. Neidig, J. Am. Chem. Soc., 2016, 138, 7492 CrossRef; (h) S. L. Daifuku, M. H. Al-Afyouni, B. E. R. Snyder, J. L. Kneebone and M. L. Neidig, J. Am. Chem. Soc., 2014, 136, 9132 CrossRef CAS PubMed; (i) M. H. Al-Afyouni, K. L. Fillman, W. W. Brennessel and M. L. Neidig, J. Am. Chem. Soc., 2014, 136, 15457–15460 CrossRef CAS PubMed; (j) S. H. Carpenter, T. M. Baker, S. B. Muñoz, W. W. Brennessel and M. L. Neidig, Chem. Sci., 2018, 9, 7931 RSC.
- T. Parchomyk and K. Koszinowski, Chem. – Eur. J., 2017, 23, 3213 CrossRef CAS PubMed.
- T. Parchomyk and K. Koszinowski, Chem. – Eur. J., 2018, 24, 16342 CrossRef CAS PubMed.
- A Mössbauer spectrum recorded at 7 K (Fig. S2†) was essentially identical to the spectrum recorded at 80 K. No signal for iron(0) nanoparticles17 or other low-valent iron species was observed by 57Fe Mössbauer spectroscopy. However, unfavourable relaxation dynamics18 may lead to pronounced line broadening that prevents detection of iron nanoparticles.
- R. B. Bedford, M. Betham, D. W. Bruce, S. A. Davis, R. M. Frost and M. Hird, Chem. Commun., 2006, 1398 RSC.
- P. Gütlich, E. Bill and A. X. Trautwein, Mössbauer Spectroscopy and Transition Metal Chemistry, Springer, Heidelberg, 2011 Search PubMed.
- A. Casitas, J. A. Rees, R. Goddard, E. Bill, S. DeBeer and A. Fürstner, Angew. Chem., Int. Ed., 2017, 56, 10108 CrossRef CAS PubMed.
- Different fits with five subspectra are possible.
- (a) S. B. Muñoz III, V. E. Fleischauer, W. W. Brennessel and M. L. Neidig, Organometallics, 2018, 37, 3093 CrossRef PubMed; (b) V. E. Fleischauer, S. B. Muñoz III, P. G. N. Neate, W. W. Brennessel and M. L. Neidig, Chem. Sci., 2018, 9, 1878 RSC; (c) K. L. Fillman, J. A. Przyojski, M. H. Al-Afyouni, Z. J. Tonzetich and M. L. Neidig, Chem. Sci., 2015, 6, 1178 RSC; (d) Y. Liu, L. Wang and L. Deng, Organometallics, 2015, 34, 4401 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, additional spectra. See DOI: 10.1039/c9dt00705a |
| This journal is © The Royal Society of Chemistry 2019 |