 Open Access Article
Open Access Article[Pb{Mn(CO)5}3][AlCl4]: a lead-manganese carbonyl with AlCl4-linked PbMn3 clusters†
Silke
Wolf
 a,
Dieter
Fenske
a,
Wim
Klopper
a,
Dieter
Fenske
a,
Wim
Klopper
 b and
Claus
Feldmann
b and
Claus
Feldmann
 *a
*a
aInstitute of Inorganic Chemistry, Karlsruhe Institute of Technology (KIT), Engesserstraße 15, 76131 Karlsruhe, Germany. E-mail: claus.feldmann@kit.edu
bInstitute of Physical Chemistry, Karlsruhe Institute of Technologie (KIT), Fritz-Haber-Weg 2, D-76131 Karlsruhe, Germany
First published on 12th March 2019
Abstract
[Pb{Mn(CO)5}3][AlCl4] containing a trigonal planar PbMn3 cluster was obtained by the reaction of PbCl2 and Mn2(CO)10 in the ionic liquid [BMIm][AlCl4]. The title compound is composed of [Pb{Mn(CO)5}3]+ carbonyl cations and [AlCl4]− anions that are connected to infinite zig-zag chains. The [Pb{Mn(CO)5}3]+ cation exhibits a central PbMn3 cluster with three equal Pb–Mn single bonds, resulting in an almost equilateral triangle of three manganese atoms with a formal Pb+I in its center. Such a cluster and compound were identified for the first time. In addition to single-crystal structure analysis, the composition, structure and properties were further characterized by density functional theory (DFT) calculations, energy dispersive X-ray spectroscopy (EDXS), and Fourier-transform infrared spectroscopy (FT-IR), as well as optical spectroscopy (UV-Vis).
Introduction
Due to their high redox stability, ionic liquids meanwhile have turned out to be versatile reaction media for the synthesis of metal clusters and reactive carbonyl compounds.1 Prominent examples include, for instance, □24Ge136 as a new modification of germanium (□ indicating non-occupied lattice sites),2 [Hg4Te8(Te2)4]8– with a porphyrin-analogous structure,3 or the Zintl-like cation [CuBi8]3+.4 In addition to the excellent redox stability of ionic liquids, their weakly coordinating properties and the inherent stabilization of compounds via cation–anion interactions are further merits.5With our studies on the reactivity of metal carbonyls, we could show that ionic liquids also represent powerful liquid media to obtain novel carbonyl compounds and clusters.1b Selected examples comprise, for instance, the adamantane-type Fe4Sn6 cluster in [{Fe(CO)3}4{SnI}6I4]2−,6a the Ge12Fe8 germanium-iron cluster in Ge12{Fe(CO)3}8(μ-I)4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6b or the anti-(WCl2)6-like [(Pb6I8){Mn(CO)5}6]2–.6c In particular, by the reaction of Fe(CO)5 with Lewis-acidic metal iodides (e.g., SnI4, GeI4), we could identify several novel carbonyl compounds that are specifically designated by the absence of alkyl and aryl ligands.6a,b,7 Such organic ligands were often considered essential for electronic and steric stabilization of carbonyl compounds.8 In this regard, the specific features of the ionic liquids allow preparation and stabilization of highly reactive carbonyl compounds and metal clusters that contain additional, even more destabilizing halogenide ligands.8
6b or the anti-(WCl2)6-like [(Pb6I8){Mn(CO)5}6]2–.6c In particular, by the reaction of Fe(CO)5 with Lewis-acidic metal iodides (e.g., SnI4, GeI4), we could identify several novel carbonyl compounds that are specifically designated by the absence of alkyl and aryl ligands.6a,b,7 Such organic ligands were often considered essential for electronic and steric stabilization of carbonyl compounds.8 In this regard, the specific features of the ionic liquids allow preparation and stabilization of highly reactive carbonyl compounds and metal clusters that contain additional, even more destabilizing halogenide ligands.8
Based on our experience with the Fe–Sn and Fe–Ge system, we have expanded our studies to the Pb–Mn system. In principle, Pb–Mn bonding is well-known for carbonyl clusters and was intensely studied in regard to the structural, magnetic, catalytic and optical properties of the resulting compounds.9 Most often isolated Pb–Mn or Mn–Pb–Mn strings were described. Larger Pb–Mn arrangements are rare and limited to Pb{![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mn(CO)2Cp}{–Mn2(CO)4Cp2} (Cp: η5-C5H4CH3).10 This compound is featured by a planar PbMn3 unit that exhibits two Pb–Mn single bonds as well as a significantly shorter Pb
Mn(CO)2Cp}{–Mn2(CO)4Cp2} (Cp: η5-C5H4CH3).10 This compound is featured by a planar PbMn3 unit that exhibits two Pb–Mn single bonds as well as a significantly shorter Pb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mn double bond. In addition, a covalent Mn–Mn bond was assumed by the authors between the two Mn atoms with Pb–Mn single bonds. As discussed above,8 halogenide-coordinated Pb–Mn clusters were considered as highly reactive and require alkyl-/aryl-ligands for electronic and steric stabilization, since halogenide ligands were described to cause a cleavage of the Pb–Mn bonds.11 This background motivated us to evaluate the feasibility of ionic liquids for obtaining novel carbonyl clusters with Pb–Mn bonding. This results in the synthesis of [Pb{Mn(CO)5}3][AlCl4] containing a [Pb{Mn(CO)5}3]+ carbonyl cation with a trigonal planar PbMn3 cluster and three equal Pb–Mn single bonds.
Mn double bond. In addition, a covalent Mn–Mn bond was assumed by the authors between the two Mn atoms with Pb–Mn single bonds. As discussed above,8 halogenide-coordinated Pb–Mn clusters were considered as highly reactive and require alkyl-/aryl-ligands for electronic and steric stabilization, since halogenide ligands were described to cause a cleavage of the Pb–Mn bonds.11 This background motivated us to evaluate the feasibility of ionic liquids for obtaining novel carbonyl clusters with Pb–Mn bonding. This results in the synthesis of [Pb{Mn(CO)5}3][AlCl4] containing a [Pb{Mn(CO)5}3]+ carbonyl cation with a trigonal planar PbMn3 cluster and three equal Pb–Mn single bonds.
Results and discussion
[Pb{Mn(CO)5}3][AlCl4] was obtained by reacting PbCl2 and Mn2(CO)10 in a mixture of [BMIm]Cl and AlCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Although, in principle, large quantities were obtained, the title compound formed only very small dark red, almost black crystals that were found on the surface of large blue, rod-shaped crystals of [Mn(CO)6][AlCl4] as a side phase. [Mn(CO)6][AlCl4] was described already,12 and its composition was confirmed here as well by single-crystal structure analysis. Taken together, the formation of the title compound can be rationalized by a redox reaction with reduction of Pb+II to Pb+I and oxidation of Mn0 to Mn+I/+II according to the following equation:
1). Although, in principle, large quantities were obtained, the title compound formed only very small dark red, almost black crystals that were found on the surface of large blue, rod-shaped crystals of [Mn(CO)6][AlCl4] as a side phase. [Mn(CO)6][AlCl4] was described already,12 and its composition was confirmed here as well by single-crystal structure analysis. Taken together, the formation of the title compound can be rationalized by a redox reaction with reduction of Pb+II to Pb+I and oxidation of Mn0 to Mn+I/+II according to the following equation:From the obtained products, Mn(CO)4Cl2 completely remained in solution, whereas [Mn(CO)6][AlCl4] shows partial crystallization. Due to its solubility and being driven by Ostwald ripening, only a few bluish crystals appear that grow to millimeter-sized individuals. The title compound [Pb{Mn(CO)5}3][AlCl4] is most insoluble in the system, and thus, formed small crystals on the surface of the large seed crystals of the co-product. Since the reactivity of [Pb{Mn(CO)5}3][AlCl4] (title compound) and [Mn(CO)6][AlCl4] (co-product) is very similar, separation is difficult and was here performed by manual separation of crystals. According to single-crystal structure determination, [Pb{Mn(CO)5}3][AlCl4] crystallizes monoclinically with the space group P21/c (Table 1, Fig. 1).
 | ||
| Fig. 1 Unit cell of [Pb{Mn(CO)5}3][AlCl4]: PbMn3 units in yellow-green, [AlCl4]− tetrahedra in grey, and C atoms and O atoms of CO ligands in light grey. | ||
| Data | [Pb{Mn(CO)5}3][AlCl4] |
|---|---|
| Sum formula | C15O15AlCl4Mn3Pb |
| Molar mass | 960.95 g mol−1 |
| Crystal system | Monoclinic |
| Space group | P21/c (no. 14) |
| Lattice parameters | a = 1020.8(2) pm |
| b = 1762.0(3)pm | |
| c = 1791.5(5)pm | |
| β = 120.9(1)° | |
| Cell volume | V = 2763.8 × 106 pm3 |
| Formula units per cell | Z = 4 |
| Calculated density | ρ = 2.309 g cm−3 |
| Measurement limits | −13 ≤ h ≤ 12, −18 ≤ k ≤ 23, −9 ≤ l ≤ 23 |
| Theta range for data collection | 3.32 to 64.34° |
| Measurement conditions | Stoe Stadivari diffractometer (Stoe) |
| λ(Ga-Kα) = 134 pm, T = 150 K | |
| Linear absorption coefficient | μ = 17.89 mm−1 |
| Number of reflections | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 417 (independent 14 417 (independent 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 261) 261) |
| Refinement method | Full-matrix least-squares on F2 |
| Merging | R int = 0.0233 |
| Number of parameters | 352 |
| Residual electron density | 3.23 to −0.82 e− 10−6 pm−3 |
| Figures of merit | R 1 (I ≥ 4σI) = 0.0325 |
| R 1 (all data) = 0.0344 | |
| wR2 (all data) = 0.0889 | |
| GooF = 0.998 |
The title compound consists of [Pb{Mn(CO)5}3]+ cations and [AlCl4]− anions. The [Pb{Mn(CO)5}3]+ cation is composed of a central Pb atom, which is connected to three Mn(CO)5 fragments (Fig. 2). Herein, Pb–Mn distances of 275.0(1) (Pb–Mn3) to 278.5(1) pm (Pb–Mn2) are observed (Table 2). Based on this equidistant connectivity and Mn–Pb–Mn angles of 117.7(1) (Mn2–Pb–Mn3) to 121.9(1)° (Mn1–Pb–Mn3), an almost ideal trigonal planar PbMn3 arrangement is formed (Fig. 2). The Pb–Mn distances clearly point to three covalent single bonds and fit well with Pb+I–Mn single bonds (279.9–281.2 pm) in [Pb6I8{Mn(CO)5}6]2–.6c Electron counting with three Pb–Mn electron-pair bonds results in three Pb-allocated electrons, and thus, a formal oxidation state of Pb+I for the group 14 element Pb. Naturally, the Pb–Mn distances in the title compound are significantly longer than the Pb0![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mn double bond (249.0 pm) in Pb{Mn(CO)2Cp}3 (Fig. 3).10 Certain increase of the Pb+I–Mn distances in [Pb{Mn(CO)5}3][AlCl4] in comparison with the Pb0–Mn single bonds (261.1–262.0 pm) in Pb{Mn(CO)2Cp}3 can be related to the steric hindrance of the CO ligands (Fig. 3).10
Mn double bond (249.0 pm) in Pb{Mn(CO)2Cp}3 (Fig. 3).10 Certain increase of the Pb+I–Mn distances in [Pb{Mn(CO)5}3][AlCl4] in comparison with the Pb0–Mn single bonds (261.1–262.0 pm) in Pb{Mn(CO)2Cp}3 can be related to the steric hindrance of the CO ligands (Fig. 3).10
 | ||
| Fig. 2 Structure of the [Pb{Mn(CO)5}3]+ cation (Pb–Mn distances in pm; ellipsoids comprise 50% of the probability density of the atoms). | ||
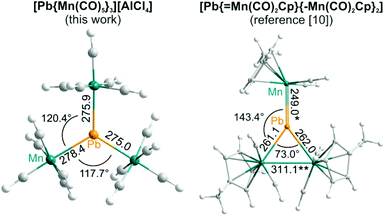 | ||
Fig. 3 Comparison of the PbMn3 cluster in [Pb{Mn(CO)5}3][AlCl4] and Pb{Mn(CO)2Cp}3 (distances in pm; *: Pb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mn double bond and **Mn–Mn single bond).10 Mn double bond and **Mn–Mn single bond).10 | ||
| Compound | Pb–Mn (pm) | Pb–Cl (pm) |
|---|---|---|
| [Pb{Mn(CO)5}3][AlCl4] (this work) | 275.0(1)–278.5(1) | 313.9(2), 334.4(2) |
| Pb{Mn(CO)2Cp}3 [10] | 261.1–262.0 (single bond) | — |
| 249.0 (double bond) | — | |
| [(Pb6I8){Mn(CO)5}6]2– [6c] | 279.9–281.2 | — |
| PbCl2 [18] | — | 284.7, 308.4 |
It needs to be noted that the PbMn3 cluster in [Pb{Mn(CO)5}3][AlCl4] is significantly different from the only known PbMn3 unit in Pb{Mn(CO)2Cp}3.10 First of all, [Pb{Mn(CO)5}3]+ contains three equalized Pb–Mn distances (Table 2), so that an almost equilateral triangle of three manganese atoms is spanned with lead in its center. In difference, Pb{Mn(CO)2Cp}3 shows largely different distances (Pb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mn: 249, Pb–Mn: 261–262 pm) and Mn–Pb–Mn angles (73.0, 143.4, 143.6°) significantly deviating from 120° (Fig. 3).10 Moreover, the title compound contains a [Pb{Mn(CO)5}3]+ cation and a positively charged PbMn3 unit with a formal Pb+I, whereas the charge neutral Pb{Mn(CO)2Cp}3 contains lead in the formal oxidation state ±0. [Pb{Mn(CO)5}3]+ with only six electrons (i.e. three Pb–Mn bonds) around the lead atom is electron deficient, for instance like BH3, and can be considered less stable than the neutral Pb{Mn(CO)2Cp}3 molecule with a filled electron octet at lead.10 In this regard, the merit of the ionic-liquid-based synthesis and its potential to obtain highly reactive compounds are again validated. The ionic-liquid-based approach is also more straightforward since simple PbCl2 can be used as the starting material, whereas Pb{Mn(CO)2Cp}3 requires an elaborate heterocumulene derivative Cp(CO)2Mn
Mn: 249, Pb–Mn: 261–262 pm) and Mn–Pb–Mn angles (73.0, 143.4, 143.6°) significantly deviating from 120° (Fig. 3).10 Moreover, the title compound contains a [Pb{Mn(CO)5}3]+ cation and a positively charged PbMn3 unit with a formal Pb+I, whereas the charge neutral Pb{Mn(CO)2Cp}3 contains lead in the formal oxidation state ±0. [Pb{Mn(CO)5}3]+ with only six electrons (i.e. three Pb–Mn bonds) around the lead atom is electron deficient, for instance like BH3, and can be considered less stable than the neutral Pb{Mn(CO)2Cp}3 molecule with a filled electron octet at lead.10 In this regard, the merit of the ionic-liquid-based synthesis and its potential to obtain highly reactive compounds are again validated. The ionic-liquid-based approach is also more straightforward since simple PbCl2 can be used as the starting material, whereas Pb{Mn(CO)2Cp}3 requires an elaborate heterocumulene derivative Cp(CO)2Mn![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Pb
Pb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mn(CO)2Cp as the starting material.10
Mn(CO)2Cp as the starting material.10
To verify the bonding situation of Pb and Mn, density functional theory (DFT) calculations were performed on a single [Pb{Mn(CO)5}3]+ cation using the TPSS functional13 in the dhf-TZVP-2c basis,14 which includes a dhf-ECP-2c relativistic effective core potential (RECP) for Pb representing its {Kr}4d104f14 core.15 The calculations were performed at the scalar-relativistic one-component (1c) and quasi-relativistic two-component (2c) levels.16
At the 1c level, scalar relativistic effects are accounted for through the spin-free part of the RECP on Pb. A geometry optimization at this level yielded a highly symmetric equilibrium structure belonging to the point group D3, in which all three Pb−Mn distances are equal (277.7 pm, Table 3). Harmonic vibrational frequencies were computed to confirm that the optimized structure is a minimum on the potential energy surface (Table 4).
| Pb−Mn (pm) | Mulliken | NPA | ||||
|---|---|---|---|---|---|---|
| Pb | Mn | Pb | Mn | |||
| [Pb{Mn(CO)5}3]+ | 1c | 277.7 | + 0.85 | −0.57 | + 0.88 | −1.13 |
| 2c | 277.7/278.1 | + 0.83 | −0.56 | + 0.82 | −1.12 | |
| Symmetry | a 1 | e | a 1 | e | e | a 1 | a 2 | e | e | a 2 |
|---|---|---|---|---|---|---|---|---|---|---|
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) (cm−1) (cm−1) |
2127 | 2094 | 2058 | 2054 | 2049 | 2044 | 2043 | 2032 | 2021 | 2010 |
| Intensity (%) | 0 | 100 | 0 | 1.4 | 0.3 | 0 | 76 | 59 | 0.1 | 0.2 |
At the 2c level, also non-scalar (i.e., spin–orbit) effects were accounted for through the 2c part of the RECP on Pb. An equilibrium structure belonging to the point group C2 was found at this level, with two equal Pb−Mn bonds of 277.7 pm and one slightly longer Pb−Mn bond of 278.1 pm. Thus, the distortion of the D3 structure towards C2 due to the spin–orbit interaction appears to be almost negligible. Harmonic vibrational frequencies were computed (from finite differences of analytical gradients) and confirmed that the optimized structure is a minimum.
In conclusion, spin–orbit effects play only a minor role in the [Pb{Mn(CO)5}3]+ cation resulting in a similar electronic structure and bonding situation in the 1c and 2c descriptions. For example, the lowest unoccupied molecular orbital (LUMO) of the 1c calculation, which is an empty Pb 6pz orbital, is virtually indistinguishable from the lowest unoccupied natural orbital obtained in the 2c-TPSS/dhf-TZVP-2c calculation (Fig. 4). The results obtained from a population analysis are also very similar at the 1c and 2c levels (Table 3). A Boys localization of the 1c occupied orbitals yielded three equivalent Pb−Mn σ bonds (Fig. 4). In summary, the bonding situation in the [Pb{Mn(CO)5}3]+ cation can be clearly described with a formal charge of +1 on Pb, an empty 6pz orbital, and three two-center-two-electron (2c2e) σ bonds. This view is in accordance with the results deduced from crystal structure analysis of the aforementioned electron-deficient character of the [Pb{Mn(CO)5}3]+ cation.
The Mn–C distances in the [Pb{Mn(CO)5}3]+ cation range from 182.5(5) (Mn2–C10) to 188.2(5) pm (Mn2–C9) and fit well with Mn–C distances of terminal CO ligands, such as in Mn2(CO)10.17 Similarly the C–O distances fit with Mn2(CO)10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 and range from 111.6(6) (C15–O15) to 113.6(6) pm (C5–O5).
17 and range from 111.6(6) (C15–O15) to 113.6(6) pm (C5–O5).
In accordance with the electron-deficient character of the [Pb{Mn(CO)5}3]+ cation, lead is further coordinated by two chlorine atoms as electron-pair donors of two different [AlCl4]− anions, so that Pb – in sum – has a distorted trigonal bipyramidal coordination of three Mn and two Cl atoms (Fig. 5). Since each [AlCl4]− anion is well coordinated to two [Pb{Mn(CO)5}3]+ cations, in sum, an infinite zig-zag chain is formed (Fig. 1 and 5). Herein, the Pb–Cl distances are not equidistant, but exhibit a shorter (Pb–Cl2: 313.9(2) pm) and a longer distance (Pb–Cl1: 334.3(2) pm) (Fig. 5). The zig-zag chains exhibit Cl–Pb–Cl angles of 173.0(1)°, and they are stacked along the crystallographic c-axis like a hexagonal rod-type packing (Fig. 1). Compared to PbCl2 (Pb–Cl: 284.7, 308.4 pm),18 the observed Pb–Cl distances are significantly increased, which is in accordance with a formal oxidation state of Pb+I and the fact that the chlorine atoms exist as [AlCl4]−, and thus, as part of a complex anion.
 | ||
| Fig. 5 Linkage of PbMn3 units via [AlCl4]− to infinite chains along the crystallographic c-axis (distances in pm; ellipsoids comprise 50% of the probability density of the atoms). | ||
The [AlCl4]− anion itself is slightly distorted from an ideal tetrahedron (Cl–Al–Cl angles: 107.0(1) to 112.5(1)°). As expected, those two Cl atoms bridging to Pb exhibit slightly longer Al–Cl distances (Al–Cl1: 215.8(2), Al–Cl2: 216.1(2) pm) compared to the two terminal Cl atoms (Al–Cl3: 210.3(2), Al–Cl4: 212.0(2) pm) (Fig. 5). This distortion points to the interaction of lead and chlorine and is often observed for asymmetric coordination (e.g. in Na[AlCl4]).19
In addition to single-crystal structure analysis, the composition, structure and properties of [Pb{Mn(CO)5}3][AlCl4] were validated by energy-dispersive X-ray spectroscopy (EDXS), Fourier-transform infrared spectroscopy (FT-IR) and optical spectroscopy (UV-Vis). Accordingly, EDXS confirms a Pb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn
Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl ratio of 1.0
Cl ratio of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6
2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.0, which fits well with the composition and the calculated values (1
4.0, which fits well with the composition and the calculated values (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4).
4).
FT-IR spectroscopy shows the characteristic CO vibrations with a strong absorption at 2098 cm−1 and three weak absorptions at 2062, 2947 and 2010 cm−1 (Fig. 6). In comparison with Mn2(CO)10,17c the CO vibrations of the title compound are slightly shifted to higher wavenumbers (Table 5), which, on the one hand, can be ascribed to the higher electronegativity of lead (EN(Pauling): 1.8) in comparison with manganese (EN(Pauling): 1.5),20 and on the other hand, to the fact that manganese carries part of the positive charge in the PbMn3 cluster. As a consequence, the observed CO vibrations are even more comparable to terminally bond carbonyl ligands in Mn(CO)5I or [Pb6I8{Mn(CO)5}6]2– (Table 5).6c,21 In addition to CO vibrations, absorptions related to the ionic liquid were observed (3152–2876, 1571, 1466 cm−1) and agree with reference spectra of the pure ionic liquid (Fig. 6). Finally, UV-Vis spectra of [Pb{Mn(CO)5}3][AlCl4] show a strong, continuous absorption above 450 nm as well as an absorption peak at 297 nm (Fig. 7). This finding is in accordance with the dark-red, almost black colour of single crystals (Fig. 7, inset).
 | ||
| Fig. 6 FT-IR spectrum of [Pb{Mn(CO)5}3][AlCl4] (green) with the ionic liquid [BMIm][Cl] shown as a reference (grey). | ||
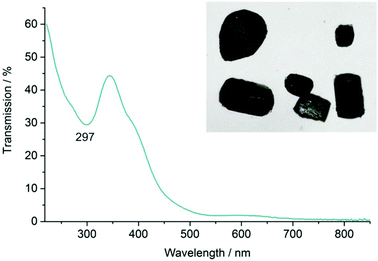 | ||
| Fig. 7 UV-Vis spectrum of [Pb{Mn(CO)5}3][AlCl4] with a photo of deep red, almost black single crystals (inset). | ||
| Compound | Vibration (cm−1) |
|---|---|
| [Pb{Mn(CO)5}3][AlCl4] (this work) | 2098, 2062, 2047, 2010 |
| Mn2(CO)10 [17c] | 2045, 2014, 1983 |
| Mn(CO)5I [21] | 2131, 2035, 2008, 1969 |
| [(Pb6I8){Mn(CO)5}6]2– [6c] | 2063, 2008, 1964 |
Conclusions
The novel carbonyl cluster compound [Pb{Mn(CO)5}3][AlCl4] is presented. The title compound was prepared by the redox reaction of PbCl2 and Mn2(CO)10 in the ionic liquid [BMIm][AlCl4] upon reduction of Pb+II to Pb+I and oxidation of Mn0 to Mn+I/+II. The [Pb{Mn(CO)5}3]+ carbonyl cation contains a central PbMn3 cluster with three Pb–Mn single bonds (275.0 to 278.4 pm), resulting in an almost equilateral triangle (Mn–Pb–Mn angles: 117.7(1) to 121.9(1)°) of three manganese atoms with a formal Pb+I in its center. Based on the crystal structure and DFT calculations, the bonding situation in the [Pb{Mn(CO)5}3]+ cation can be described with a formal charge of +1 on Pb, an empty 6pz orbital, and three two-center-two-electron (2c2e) σ bonds. Similar to BH3, thus, [Pb{Mn(CO)5}3]+ represents an electron-deficient species. Such a cluster and compound were identified for the first time.Beside the three Mn(CO)5 units, the electron-deficient Pb center is further coordinated by two Cl atoms of [AlCl4]− anions serving as electron-pair donors. Together with the triangular PbMn3 cluster unit, this results in a trigonal bipyramidal coordination around Pb. Due to bidentate coordination of the [Pb{Mn(CO)5}3]+ carbonyl cations as well as of the [AlCl4]− anions, finally, infinite zig-zag chains along the crystallographic c-axis are formed.
The successful synthesis of the reactive carbonyl cluster can be considered as another example of the advantage of ionic-liquid-based synthesis and its promising features such as the weakly coordinating properties and the redox stability of the ionic liquids.
Experimental
Synthesis
Analytical equipment
Fourier-transform infrared (FT-IR) spectra were recorded on a Bruker Vertex 70 FT-IR spectrometer (Bruker). The samples were measured as pellets in KBr. Thus, 300 mg of dried KBr and 0.5–1.0 mg of the sample were carefully pestled together and pressed to a thin pellet.
Energy dispersive X-ray (EDX) analysis was performed by using an Ametec EDAX mounted on a Zeiss SEM Supra 35 VP scanning electron microscope. Samples were prepared in a glovebox by selecting single crystals that were fixed on conductive carbon pads on aluminum sample holders. Samples were handled under inert conditions during transport and sample preparation.
Optical spectroscopy (UV-Vis) of powder samples was performed on a Shimadzu UV-2700 spectrometer, equipped with an integrating sphere, in a wavelength interval of 250–800 nm against BaSO4 as the reference.
Computation
Density functional theory (DFT) calculations were performed using the TPSS functional13 in the dhf-TZVP-2c basis,14 which includes a dhf-ECP-2c relativistic effective core potential (RECP) for Pb representing its {Kr}4d104f14 core.15 Version 7.4 of the TURBOMOLE program package was used.25 Calculations were performed at the scalar-relativistic one-component (1c) and quasi-relativistic two-component (2c) levels.16Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the Deutsche Forschungsgemeinschaft (DFG) for funding in the Priority Program SPP1708 “Synthesis near room temperature”.Notes and references
- (a) P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2008 Search PubMed; (b) D. Freudenmann, S. Wolf, M. Wolff and C. Feldmann, Angew. Chem., Int. Ed., 2011, 50, 11050 CrossRef CAS PubMed (Review). (c) M. F. Groh, A. Wolff, M. A. Grasser and M. Ruck, Int. J. Mol. Sci., 2016, 17, 1452 CrossRef PubMed.
- A. M. Guloy, R. Ramlau, Z. Tang, W. Schnelle, M. Baitinger and Y. Grin, Nature, 2006, 443, 320 CrossRef CAS PubMed.
- (a) S. Santner and S. Dehnen, Inorg. Chem., 2015, 54, 1188 CrossRef CAS PubMed; (b) R. J. Wilson, F. Hastreiter, K. Reiter, P. Büschelberger, R. Wolf, R. Gschwind, F. Weigend and S. Dehnen, Angew. Chem., Int. Ed., 2018, 57, 15359 CrossRef CAS PubMed.
- M. Knies, M. Kaiser, A. Isaeva, U. Müller, T. Doert and M. Ruck, Chem. – Eur. J., 2018, 24, 127 CrossRef CAS PubMed (Review).
- I. M. Riddlestone, A. Kraft, J. Schaefer and I. Krossing, Angew. Chem., Int. Ed., 2018, 57, 13982 CrossRef CAS PubMed.
- (a) S. Wolf, F. Winter, R. Pöttgen, N. Middendorf, W. Klopper and C. Feldmann, Chem. – Eur. J., 2012, 18, 13600 CrossRef CAS PubMed; (b) S. Wolf, W. Klopper and C. Feldmann, Chem. Commun., 2018, 54, 1217 RSC; (c) S. Wolf, K. Reiter, F. Weigend, W. Klopper and C. Feldmann, Inorg. Chem., 2015, 54, 3989 CrossRef CAS PubMed.
- (a) S. Wolf, F. Winter, R. Pöttgen, N. Middendorf, W. Klopper and C. Feldmann, Dalton Trans., 2012, 41, 10605 RSC; (b) S. Wolf and C. Feldmann, Z. Anorg. Allg. Chem., 2017, 643, 25 CrossRef CAS.
- P. Braunstein, L. A. Oro and P. R. Raithby, Metal Clusters in Chemistry, Wiley-VCH, Weinheim, 2008 Search PubMed.
- (a) F. Ettel, G. Hutter and L. Zsolnai, Angew. Chem., Int. Ed. Engl., 1989, 28, 1496 CrossRef; (b) F. Ettel, M. Schollenberger, B. Schiemenz, W. Imhof, G. Huttner and L. J. Zsolnai, Organomet. Chem., 1994, 476, 207 CrossRef CAS; (c) F. Ettel, M. Schollenberger, B. Schiemenz, G. Huttner and L. J. Zsolnai, Organomet. Chem., 1994, 476, 153 CrossRef CAS; (d) V. S. Leong and N. J. Cooper, Organometallics, 1988, 7, 2080 CrossRef CAS.
- H.-J. Kneuper, E. Herdtweck and W. A. Herrmann, J. Am. Chem. Soc., 1987, 109, 2508–2509 CrossRef CAS.
- (a) H. J. Haupt, W. Schubert and F. Huber, J. Organomet. Chem., 1973, 54, 231 CrossRef CAS; (b) M. R. Brooth, D. J. Cardin, N. A. D. Carey, H. C. Clark and B. R. Sreenathan, J. Organomet. Chem., 1970, 21, 171 CrossRef.
- (a) E. O. Fischer and K. Öfele, Angew. Chem., 1961, 73, 581 CrossRef CAS; (b) E. O. Fischer, K. Fichtel and K. Öfele, Chem. Ber., 1962, 95, 249 CrossRef CAS.
- J. Tao, J. P. Perdew, V. N. Staroverov and G. E. Scuseria, Phys. Rev. Lett., 2003, 91, 146401 CrossRef PubMed.
- M. K. Armbruster, W. Klopper and F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 4862 RSC.
- B. Metz, H. Stoll and M. Dolg, J. Chem. Phys., 2000, 113, 2563 CrossRef CAS.
- M. K. Armbruster, F. Weigend, C. van Wüllen and W. Klopper, Phys. Chem. Chem. Phys., 2008, 10, 1748 RSC.
- (a) L. F. Dahl and R. E. Rundle, Acta Crystallogr., 1963, 16, 419 CrossRef CAS; (b) M. R. Churchill, K. N. Amoh and H. J. Wasserman, Inorg. Chem., 1981, 20, 1609 CrossRef CAS; (c) H. Haas and R. K. J. Sheline, Chem. Phys., 1967, 47, 2996 CAS.
- M. Lumbreras, J. Protas, S. Jebbari, G. J. Dirksen and J. Schoonman, Solid State Ionics, 1986, 20, 295 CrossRef CAS.
- W. Scheinert and A. Weiss, Z. Naturforsch., A: Phys., Phys. Chem., Kosmophys., 1976, 31, 1354 Search PubMed.
- J. E. Huheey, E. A. Keiter, R. L. Keiter and O. K. Medhi, Inorganic Chemistry: Principles of Structure and Reactivity, Pearson, Cambridge, 2008 Search PubMed.
- H. Li and I. S. Butler, Appl. Spectrosc., 1992, 46, 1785 CrossRef CAS.
- J. Koziskova, F. Hahn, J. Richter and J. Kozisek, Acta Chim. Slovaca, 2016, 9, 136 CAS.
- (a) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed; (b) G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3 Search PubMed.
- DIAMOND Version 4.2.2: Crystal and Molecular Structure Visualization, Crystal Impact GbR, Bonn, 2016 Search PubMed.
- TURBOMOLE V7.4 2019, a development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, since 2007; available from http://www.turbomole.com.
Footnote |
| † CCDC 1890307. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c9dt00309f |
| This journal is © The Royal Society of Chemistry 2019 |


