Structural and thermodynamic aspects of hydration of Gd(iii) systems†
Abstract
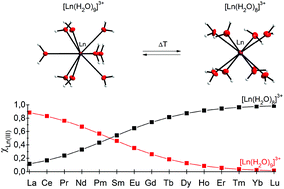
X-ray crystal structures of Gd(III) and Lu(III) aqua ions as well as their complexes with polyaminopolycarboxylates (EDTA, CDTA, EGTA, DTPA, DOTA) were determined: [Gd(H2O)9](CF3SO3)3, [Gd(H2O)8]Cl3·C10H20O5, [Lu(H2O)8]Cl3·C12H24O6·4H2O, [C(NH2)3][Gd(EDTA)(H2O)3], [C(NH2)3]2[Lu(EDTA)(H2O)2]ClO4·6H2O, [C(NH2)3][Lu(CDTA)(H2O)2]·6H2O, [C(NH2)3][Gd(EGTA)(H2O)]·2H2O, [C(NH2)2(N2H4)][Gd(HDTPA)(H2O)]·2H2O, Na[Gd(DOTA)(H2O)]·4H2O, and K2[Lu(DOTA)]Cl·4.6H2O. The weighted sums of UV absorption spectra of appropriate crystals were used to reproduce the spectra of the Gd(III) aqueous solutions in the temperature range 276–363 K. It was shown that in aqueous solution the Gd(III)-EGTA, Gd(III)-DTPA and Gd(III)-DOTA complexes exist as almost pure monohydrate [GdL(H2O)]n− species, while in the case of the Gd(III) aqua ion, Gd(III)-EDTA and Gd(III)-CDTA systems the equilibria between variously hydrated species were found. The derived molar fractions of these species were used to determine the ΔG, ΔH and ΔS of hydration. It was shown that these thermodynamic functions may be derived not only from the spectra of the hypersensitive transitions, but from other f–f transitions as well. Next the ΔG, ΔH and ΔS values of hydration for the other Ln(III)-EDTA systems (where Ln = Pr, Nd, Sm, Eu) were determined. It was found that the ΔG298 values of the dehydration reaction for Ln(III)-EDTA complexes (where Ln = Pr, Nd, Sm, Eu, Gd, Ho, Er) were almost linearly dependent on the number of 4f electrons in the whole series of lanthanides. Moreover, it was shown that the point, where the ratio of [LnL(H2O)n] : [LnL(H2O)n−1] is equal to 1, shifts along the lanthanide series depending on the ligand denticity – the higher the ligand denticity, the farther the point of the equimolar ratio in the lanthanide series. The presented results are the first systematic experimental study on the thermodynamic description of the hydration equilibrium of Gd(III) compounds.


 Please wait while we load your content...
Please wait while we load your content...