Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electrophilic boron carboxylate and phosphinate complexes†
Diya
Zhu
a,
James H. W.
LaFortune
a,
Rebecca L.
Melen
 *b and
Douglas W.
Stephan
*b and
Douglas W.
Stephan
 *a
*a
aDepartment of Chemistry, University of Toronto, M5S3H6, Canada. E-mail: dstephan@chem.utoronto.ca
bSchool of Chemistry, Cardiff University, Main Building, Park Place, Cardiff, CF10 3AT, Cymru/Wales, UK
First published on 9th January 2019
Abstract
The reactions of a series of carboxylic acids with H2B(C6F5)·SMe2 are shown to afford species of the form [RC(O)OB(C6F5)]2O, (R = Tol 1, Ph 2, C6F53, Me2BrC 4, Me 5) in 87–95% yields with the concurrent reduction of the carboxylic acid to the corresponding aldehyde. A mechanism for the formation of 1–5 is proposed to proceed via a cyclic eight-membered ring species. Analogues of these species were prepared via reactions of carboxylic and phosphinic acids with HB(C6F5)2 and H2B(C6F5)·SMe2, respectively, to give [TolC(O)OB(C6F5)2]26, [(C6F5)C(O)OB(C6F5)2]27, and [Ph2P(O)OBH(C6F5)]28. These products react subsequently to give TolC(O)OBH(C6F5)(NC5H4NMe2) 9 and Ph2P(O)OBH(C6F5)(NC5H4NMe2) 10. The acyloxyborate derivatives 1–4 were shown to be inactive in mediating the direct amidation of carboxylic acids, consistent with previous observations that infer the need for a sterically congested environment about the boron centres.
Introduction
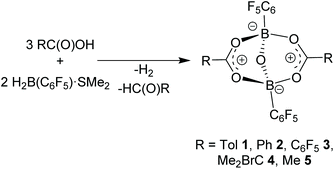
The formulation of acyloxyboranes generated a lot of confusion in the early literature, specifically over the formulation of the product derived from the reaction of orthoboric acid and acetic anhydride. Originally thought to be boron acetate, this notion was revised in 1957 when Hayter et al.1 correctly formulated the product as the acyloxyborate [RC(O)OBR′]2O (Scheme 1). Subsequently, a number of related studies have provided structural data or established new protocols to such species. These include reports by the groups of Lappert,2 Perotti,3 Sporzyński,4 Köster,5 and Wrackmeyer.6 Despite the relatively few studies of these compounds, acyloxyborates have found important applications in fire-proofing polymer compositions, as stabilisers for synthetic rubbers, and in the pharmaceutical industry.7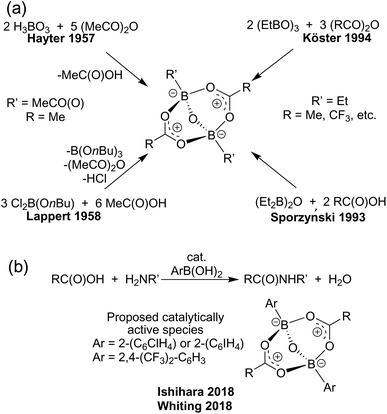 | ||
| Scheme 1 (a) Selected synthetic routes to acyloxyborate derivatives. (b) Direct amidation catalysis by acyloxyborates. | ||
Related boron carboxylate species have also been proposed as intermediates in dehydrative condensations between a carboxylic acid and an amine using catalysts derived from borate esters8–10 as well as boric,11 boronic,12–21 and borinic acids.22 Recently however, Whiting and coworkers23 have suggested an alternative mechanism in which an oxo-bridged bis-boron species, i.e. an acyloxyborate, acts as the catalytically active species for direct amidation reactions (Scheme 1). In this catalysis, Whiting23 showed that acyloxyborates are generated in situ by the reaction of boronic and carboxylic acids in the presence of molecular sieves, although these authors were able to prepare related species directly from the reaction of an arylboronic acid and phenylacetic acid. The intermediacy of these acyloxyborates was further supported by Ishihara and coworkers,24 who showed that ortho-substituents on the boron-bound aryl group prevented the coordination of amine to boron, thus accelerating the catalysis.
Our interest in boron-based species that incorporate electron withdrawing substituents has prompted us to probe related carboxylate derivatives. As is well established that borohydride reductions of carboxylic acids proceed via boron-carboxylate species,25,26 we were interested in probing the reactions of carboxylic acids with the electrophilic boranes. In the initial effort, we previously reported the preparation of the salt [Cp*2Fe][PhCO2B(C6F5)3] via the one electron reduction of a peroxide in the presence of a borane.27,28 Herein, we describe the reactions of H2B(C6F5)·SMe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 and HB(C6F5)2 with carboxylic and phosphinic acids. Generally, these reactions result in eight-membered cyclic products, while the reactions of H2B(C6F5)·SMe2 and carboxylic acids provide a facile route to bicyclic acyloxyborate derivatives with concurrent acid-reduction to aldehyde. Experimental and computational data support a proposed mechanism for the formation of the latter products. The catalytic utility of these acyloxyborate species in the direct amidation reactions is also probed.
29 and HB(C6F5)2 with carboxylic and phosphinic acids. Generally, these reactions result in eight-membered cyclic products, while the reactions of H2B(C6F5)·SMe2 and carboxylic acids provide a facile route to bicyclic acyloxyborate derivatives with concurrent acid-reduction to aldehyde. Experimental and computational data support a proposed mechanism for the formation of the latter products. The catalytic utility of these acyloxyborate species in the direct amidation reactions is also probed.
Results and discussion
The reaction of p-toluic acid with Lancaster's reagent, H2B(C6F5)·SMe2,29 was performed in DCM at room temperature prompting the evolution of H2. Repeated reactions showed that all the reagents were consumed when combined in an acid to borane ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The 11B NMR spectrum revealed the complete conversion of H2B(C6F5)·SMe2 into a new four-coordinate boron species that exhibited a broad resonance at 5.1 ppm. The 19F{1H} NMR spectrum showed a gap between the resonances attributed to the meta- and para-fluorine atoms of the perfluorinated arene rings (Δδ = 8.3 ppm), consistent with the presence of a four-coordinate boron centre. After work-up, a white solid 1 was isolated in 95% yield (Scheme 2). Single crystals for X-ray diffraction analysis were obtained through diffusion of pentane into a benzene solution at ambient temperature (Fig. 1). The solid-state structure showed that 1 was [TolC(O)OB(C6F5)]2O, a [3,3,1] bicycle in which two boron centres are linked by a bridging oxygen atom and two carboxylate ligands.
2. The 11B NMR spectrum revealed the complete conversion of H2B(C6F5)·SMe2 into a new four-coordinate boron species that exhibited a broad resonance at 5.1 ppm. The 19F{1H} NMR spectrum showed a gap between the resonances attributed to the meta- and para-fluorine atoms of the perfluorinated arene rings (Δδ = 8.3 ppm), consistent with the presence of a four-coordinate boron centre. After work-up, a white solid 1 was isolated in 95% yield (Scheme 2). Single crystals for X-ray diffraction analysis were obtained through diffusion of pentane into a benzene solution at ambient temperature (Fig. 1). The solid-state structure showed that 1 was [TolC(O)OB(C6F5)]2O, a [3,3,1] bicycle in which two boron centres are linked by a bridging oxygen atom and two carboxylate ligands.
 | ||
| Fig. 1 POV-ray depictions of (a) 1, (b) 2, and (c) 3. C: black, O: red, B: yellow-green, and F: pink. Hydrogen atoms have been omitted for clarity. | ||
In a similar fashion, benzoic acid, pentafluorobenzoic acid, 2-bromo-2-methylpropionic acid, and acetic acid reacted with H2B(C6F5)·SMe2 to give the products formulated as [RC(O)OB(C6F5)]2O (R = Ph 2, C6F53, Me2BrC 4, Me 5) in 87–90% yields (Scheme 1). The spectroscopic data for these compounds were similar to those described for 1. Crystallographic studies also confirmed the formulations of 2 and 3 (Fig. 1).
The structural data for 1–3 confirmed a pseudo-tetrahedral geometry about the two boron centres linked by an oxygen atom and bridged by two carboxylate units. The B–O–B fragments exhibit B–O bond lengths of 1.398(2) and 1.401(2) Å in 1, 1.401(3) and 1.402(3) Å in 2, and 1.394(4) and 1.400(4) Å in 3. The corresponding B–O–B angles were found to be 110.6(2)°, 110.5(2)°, and 112.9(2)°, respectively. The B–O bond distances for the carboxylic oxygen atoms in these species were found to fall in the range of 1.522(3) to 1.612(3) Å. The B–C bond lengths in 1 and 2 ranged from 1.602(3) to 1.612(3) Å, while those in 3 were found to be 1.595(4) and 1.582(4) Å. The shorter B–C bonds in 3 are consistent with the presence of the electron withdrawing carboxylates. Nonetheless, these general structural features are similar to those previously reported for the diacyloxy B–O–B species [(MeC(O)O)2B]2O,3 [MeC(O)OBEt]2O,5 and [MeC(O)OBCy]2O6 as reported by Perotti, Köster, and Wrackmeyer, respectively.
In addition to H2, the second by-product in the formation of 1–5 was identified as the aldehyde derived from the reduction of the starting carboxylic acid. Examination of the reaction mixture by DART-MS confirmed the formation of the corresponding aldehyde (for details see the ESI†). In addition, the 1H NMR spectrum of the reaction mixture of compound 1 showed a broad resonance ca. 12 ppm, supporting the presence of the corresponding aldehyde.
The mechanism of the formation of 1–5 is thought to be initiated by protonolysis generating boryl-ester. Dimerization of this species is consistent with the electrophilic nature of the boron centre. In the subsequent reaction with a third equivalent of acid, its carbonyl fragment is in close proximity to a boron hydride, which presumably results in the liberation of aldehyde and the formation of the oxo-bridge between the two boron centres (Scheme 3).
In support of the proposed initial dimerization in this mechanism, a related product was derived from the reaction of p-toluic acid with Piers’ borane, HB(C6F5)2.30 This reaction afforded the near quantitative formation of a product formulated as [TolC(O)OB(C6F5)2]26 (Scheme 4). The 19F NMR spectrum showed resonances at −135.1, −154.0, and −162.1 ppm, while the 11B NMR signal was observed at 5.2 ppm. These data are consistent with four-coordinate boron centres and this was confirmed crystallographically (Fig. 2). The structure demonstrates the eight-membered ring in which the carboxylic units bridge the two boron centres. This species is structurally similar to dialkyl-group 13 carboxylates reported by Justyniak et al.31 as well as the species [Ph2PO2B(C6F5)2]2.32 Overall, this ring adopts a pseudo-boat conformation in which the C6F5 rings on each of the boron atoms are oriented so as to permit π-stacking. This observation appears to be a solid-state packing effect as this inequivalence of the fluoroarene rings is not reflected in the 19F NMR spectral data.
 | ||
| Fig. 2 POV-ray depiction of 6. C: black, O: red, B: yellow-green, and F: pink. All hydrogen atoms have been omitted for clarity. | ||
In a similar fashion, the corresponding reaction of pentafluorobenzoic acid with Piers’ borane, HB(C6F5)2, afforded the product formulated as [(C6F5)C(O)OB(C6F5)2]27 in 83% isolated yield (Scheme 4). While the 19F and 11B NMR data were consistent with the formulation of 7, its poor solubility precluded the acquisition of 13C data.
In an analogous reaction, the combination of stoichiometric amounts of H2B(C6F5)·SMe2 and Ph2P(O)OH was performed. This led to the release of H2 and the formation of a clear and colourless solution after 12 h (Scheme 4). A colourless crystalline solid 8 was isolated in 68% yield after workup. Dissolution of 8 in CDCl3 gives rise to two 31P signals at 40.8 and 37.6 ppm in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.44 ratio. Similarly, two sets of pentafluorophenyl resonances in the 19F NMR spectrum suggest the presence of two four-coordinate boron environments. Nonetheless, only a single, broad 11B NMR signal was seen at 0.3 ppm while the corresponding 1H{11B} NMR spectrum showed two broad resonances at 4.18 and 3.91 ppm attributed to two B–H environments. To probe the possibility of a monomer/dimer equilibrium (Scheme 4), a variable temperature multinuclear NMR study was performed on 8. Upon cooling to −30 °C, the ratio of the two resonances at 40.8 and 37.6 ppm was altered slightly to 1
0.44 ratio. Similarly, two sets of pentafluorophenyl resonances in the 19F NMR spectrum suggest the presence of two four-coordinate boron environments. Nonetheless, only a single, broad 11B NMR signal was seen at 0.3 ppm while the corresponding 1H{11B} NMR spectrum showed two broad resonances at 4.18 and 3.91 ppm attributed to two B–H environments. To probe the possibility of a monomer/dimer equilibrium (Scheme 4), a variable temperature multinuclear NMR study was performed on 8. Upon cooling to −30 °C, the ratio of the two resonances at 40.8 and 37.6 ppm was altered slightly to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.56. These data suggest a dynamic equilibrium in which a dimer formulated as [Ph2P(O)OBH(C6F5)]2 dissociates to a monomeric species. Subsequently, single crystals of 8 suitable for X-ray diffraction analysis were obtained by cooling a saturated dichloromethane solution of 8 at −35 °C. The solid-state structure revealed a dimeric structure with the expected eight-membered ring, adopting a pseudo-chair conformation (Fig. 3). The B–O and P–O bond distances were found to be on average 1.438(6) and 1.525(3) Å, respectively.
0.56. These data suggest a dynamic equilibrium in which a dimer formulated as [Ph2P(O)OBH(C6F5)]2 dissociates to a monomeric species. Subsequently, single crystals of 8 suitable for X-ray diffraction analysis were obtained by cooling a saturated dichloromethane solution of 8 at −35 °C. The solid-state structure revealed a dimeric structure with the expected eight-membered ring, adopting a pseudo-chair conformation (Fig. 3). The B–O and P–O bond distances were found to be on average 1.438(6) and 1.525(3) Å, respectively.
 | ||
| Fig. 3 POV-ray depiction of 8. C: black, O: red, B: yellow-green, F: pink, P: orange, and H: white. All hydrogen atoms except those on boron have been omitted for clarity. | ||
The nature of 8 is analogous to the proposed intermediate Int, in the above reactions with carboxylic acids. However, in contrast to the carboxylic acid analogues, efforts to react 8 with an excess amount of diphenylphosphinic acid or with carboxylic acids failed. In addition, 8 did not react with 1,4-pentadiene in toluene even on heating to 110 °C for 12 h. The latter result is consistent with the strong basicity of the phosphinic acid fragment that binds to boron, precluding the generation of a transient three-coordinate boron centre necessary for hydroboration.
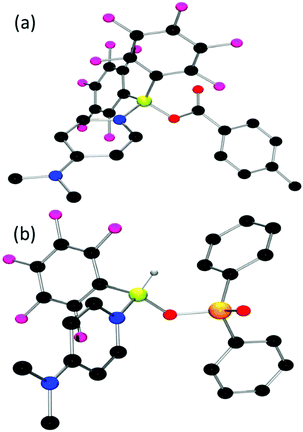
While the NMR data for 8 suggests a monomer/dimer equilibrium, the corresponding data for 6 indicate a robust eight-membered ring. Nonetheless, the reaction of 6 and 8 with DMAP resulted in the formation of new species 9 and 10, respectively. In the case of 9, the 19F NMR spectrum showed signals at −133.5, −157.3, and −163.6 ppm while the 11B resonance was seen at 1.6 ppm, consistent with a four-coordinate boron centre. A similar conclusion was drawn for 10 based on the 19F NMR signals at −134.9, −158.1, and −164.0 ppm, with the 11B signal at 0.8 ppm. The 31P NMR resonance for 10 was seen at 25.3 ppm. These data suggest the coordination of DMAP to the boron centres of 9 and 10, resulting in products TolCO2BH(C6F5)(NC5H4NMe2) and Ph2PO2BH(C6F5)(NC5H4NMe2), respectively (Scheme 5). These formulations were confirmed crystallographically (Fig. 4). The B–O distances in 9 and 10 were found to be 1.484(3) and 1.485(3) Å, while the B–N distances were 1.605(3) and 1.595(4) Å, respectively.
To probe the stability of 8 further, DFT computations were performed at the M062X/def2-TZVPP level of theory with GD3 dispersion and PCM modelling of dichloromethane solvation. Given that 8 is the phosphinic acid analogue of the proposed intermediate, Int1 (Scheme 2), in the formation of 1–5, the optimized structures of 8 and Int1 were computed. Interestingly, the lowest energy conformation of 8 is one in which the hydride atoms on boron adopt a transoid conformation. In contrast, for Int1 the orientation in which the hydride atoms are cisoid is lower in energy. This difference is attributed to the minimization of steric conflict in 8. The HOMO for 8 is located on the fluoroarene rings, whereas the hydride atoms on boron in Int1 contributed to the HOMO (Fig. 5).
Finally, as Whiting has proposed that the active catalysts in their direct amidation reactions are structurally analogous to 1–5, the utility of the present compounds was probed. Addition of two equivalents of aniline to the species 1–4 generated insoluble products that could not be characterised. However, the corresponding reaction of 5 gave soluble products and showed the generation of a trace amount of amide after 12 h as evidenced by the 1H NMR spectrum (see the ESI†). The corresponding 11B NMR spectrum revealed the consumption of 5 and the appearance of two signals at 3 and 18 ppm. These results were reminiscent of data presented by Whiting23 and Ishihara24 for analogous species with sterically unencumbered boron centres. Although these products could not be isolated from the present reaction, the inability of 5 to catalyse amide formation is consistent with the need for steric hindrance about the boron atom to prompt nucleophilic attack at the carbon of a bridging carboxylic group, thereby prompting the amidation pathway as proposed by Whiting and Ishihara. This notion is also supported by the formation of 9 and 10 which illustrates the ready access of a donor to the respective boron sites in 6 and 8.
Conclusions
In summary, synthetic methods to new acyloxyborates of the formula [RC(O)OB(C6F5)]2O (1–5) are derived from the reactions of H2B(C6F5)·SMe2 with carboxylic acids. These species are formed via reactions involving protonolysis of the B–H bonds and reduction of carboxylic acid to generate the corresponding aldehyde. The mechanism is proposed to proceed via a cyclic intermediate that dissociates and allows the B–H bonds to react with an additional equivalent of carboxylic acid. This proposed intermediate is analogous to the cyclic species 6–8. For the latter species, the lack of steric effects of the ortho-substituent on the boron atoms results in dimer cleavage and facile binding of donors to the boron centres affording 9 and 10. These observations together with the ineffective catalytic activity of 1–5 in direct amidation reactions are consistent with the previous inference of the need for steric congestion to generate a catalytically active acyloxyborate.23,24 Efforts to use boron carboxylate derivatives as catalysts in a variety of reactions are the subject of ongoing study.Experimental section
General remarks
All reactions and work-up procedures were performed under an inert atmosphere of dry, oxygen-free N2 by means of standard Schlenk techniques or glovebox techniques (MBRAUN glovebox equipped with a −35 °C freezer) unless otherwise specified. All glassware was oven-dried and cooled under vacuum before use. Dichloromethane (DCM) was distilled over CaH2. Pentane was collected from a Grubbs-type column system manufactured by Innovative Technology and degassed. Solvents were stored over activated 4 Å molecular sieves. Molecular sieves, type 4 Å (pellets, 3.2 mm diameter), purchased from Sigma Aldrich were activated prior to usage by iteratively heating under vacuum for 24 hours. CDCl3 purchased from Cambridge Isotope Laboratories was vacuum distilled, further degassed, and stored over activated 4 Å molecular sieves in a glovebox for at least 8 hours prior to use. Unless otherwise mentioned, chemicals were purchased from Sigma Aldrich or TCI. Lancaster's reagent H2B(C6F5)·SMe2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 and Piers’ borane HB(C6F5)2
29 and Piers’ borane HB(C6F5)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 were prepared using literature methods. NMR spectra were recorded at room temperature (298 K) unless otherwise mentioned on a Bruker Avance III 400 MHz, an Agilent DD2 400, and an Agilent DD2 500. Spectra were referenced to the residual solvent signals (CDCl3: 1H = 7.26; 13C = 77.2 ppm; toluene-d8: 1H = 7.09, 7.01, 6.97, and 2.08 ppm and 13C = 137.48, 128.87, 17.96, 125.13, and 20.43 ppm). Chemical shifts (δ) are reported in ppm and coupling constants (J) are listed as absolute values in Hz. Multiplicities are reported as singlet (s), doublet (d), triplet (t), multiplet (m), overlapping (ov), and broad (br). High-resolution mass spectra (HRMS) were obtained on a JMS-T100LC JOEL DART mass spectrometer. Elemental analyses for C, H, and N were performed by ANALEST (University of Toronto) employing a PerkinElmer 2400 Series II CHNS Analyser.
30 were prepared using literature methods. NMR spectra were recorded at room temperature (298 K) unless otherwise mentioned on a Bruker Avance III 400 MHz, an Agilent DD2 400, and an Agilent DD2 500. Spectra were referenced to the residual solvent signals (CDCl3: 1H = 7.26; 13C = 77.2 ppm; toluene-d8: 1H = 7.09, 7.01, 6.97, and 2.08 ppm and 13C = 137.48, 128.87, 17.96, 125.13, and 20.43 ppm). Chemical shifts (δ) are reported in ppm and coupling constants (J) are listed as absolute values in Hz. Multiplicities are reported as singlet (s), doublet (d), triplet (t), multiplet (m), overlapping (ov), and broad (br). High-resolution mass spectra (HRMS) were obtained on a JMS-T100LC JOEL DART mass spectrometer. Elemental analyses for C, H, and N were performed by ANALEST (University of Toronto) employing a PerkinElmer 2400 Series II CHNS Analyser.
X-ray diffraction studies
Single crystals were coated with paratone oil, mounted on a cryoloop and frozen under a stream of cold nitrogen. Data were collected on a Bruker Apex2 X-ray diffractometer at 150(2) K for all crystals using graphite monochromated Mo-Kα radiation (0.71073 Å). Data were collected using Bruker APEX-2 software and processed using SHELX and an absorption correction applied using multi-scan within the APEX-2 program. All structures were solved and refined by direct methods within the SHELXTL package.2: Yield: 228.3 mg (90% isolated yield). 1H{19F} NMR (400 MHz, CDCl3): δ 8.27 (d, J = 7.9 Hz, 4H), 7.78 (t, J = 7.5 Hz, 2H), 7.57 (t, J = 7.7 Hz, 4H). 19F{1H} NMR (377 MHz, CDCl3): δ −134.5 (m, 3JFF = 22.6 Hz, 4F, o-C6F5), −154.74 (t, 3JFF = 20.3 Hz, 2F, p-C6F5), −163.2 (m, 3JFF = 21.1 Hz, 4F, m-C6F5). 11B NMR (128 MHz, CDCl3): δ 4.8. 13C{1H} NMR (126 MHz, CDCl3): δ 176.4, 145.4 (dm, 1JFC = 247 Hz), 141.3 (dm, 1JFC = 258 Hz), 137.3 (dm, 1JFC = 250 Hz), 137.0, 131.5, 129.2, 126.5. The resonance for the ipso-B(C6F5) carbon was not observed. Elemental analysis: Calc.: C 50.86%, H 1.64%; Exp.: C 50.78%, H 1.50%.
3: Yield: 295.0 mg (90% isolated yield). 19F{1H} NMR (377 MHz, CDCl3): δ −130.6 (br, 4F, o-C6F5), −134.31 (d, 3JFF = 22.9 Hz, 4F, o-C6F5), −137.5 (br, 2F, p-C6F5), −153.1 (t, 3JFF = 20.2 Hz, 2F, p-C6F5), −157.7 (br, 4F, m-C6F5), −162.68 (m, 3JFF = 18.9 Hz, 4F, m-C6F5). 11B NMR (128 MHz, CDCl3): δ 4.2. The 13C NMR spectrum was not observed due to the poor solubility of compound 3. Elemental analysis: Calc.: C 39.34%; Exp.: C 38.70%.
4: Yield: 55.1 mg (88% isolated yield). 1H NMR (400 MHz, toluene-d8): δ 1.61 (s, 12 H, CH3). 19F{1H} NMR (377 MHz, toluene-d8): δ −134.4 (d, 3JFF = 23.7 Hz, 4F, o-C6F5), −153.7 (t, 3JFF = 20.2 Hz, 2F, p-C6F5), −162.7 (m, 3JFF = 22.6 Hz, 4F, m-C6F5). 11B NMR (128 MHz, toluene-d8): δ 5.4. 13C NMR (126 MHz, toluene-d8): δ 185.1, 149.1 (dm, 1JFC = 246 Hz), 141.8 (dm, 1JFC = 247 Hz), 137.7 (dm, 1JFC = 249 Hz), 53.8, 28.32. The resonance for the ipso-B(C6F5) carbon was not observed. Elemental analysis: Calc.: C 34.14%, H 1.72%; Exp.: C 33.84%, H 1.55%.
5: Yield: 180.3 mg (89% isolated yield). 1H NMR (400 MHz, toluene-d8): δ 1.45 (s, 6 H, CH3). 19F NMR (377 MHz, toluene-d8): δ −134.7 (d, 3JFF = 16.2, 4F, o-C6F5), −154.7 (t, 3JFF = 20.5 Hz, 2F, p-C6F5), −163.5 (m, 4F, m-C6F5). 11B NMR (128 MHz, toluene-d8): δ 4.2. The 13C NMR spectrum was not observed due to the poor solubility of compound 5. Elemental analysis: Calc.: C 39.23%, H 1.23%; Exp.: C 39.02%, H 1.25%.
7: Yield: 79 mg (83% isolated yield). 19F{1H} NMR (470 MHz, toluene-d8): δ 133.6 (4F, B-o-C6F5), −135.6, −150.8, −157.7 (2F, B-p-C6F5), −161.4 (4F, B-m-C6F5). 11B NMR (160 MHz, toluene-d8): δ 1.5. 13C{1H} NMR (126 MHz, CDCl3): δ was not observed due to poor solubility of compound 7. Elemental analysis: Calc.: 41.05%; Exp.: C 41.77%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. Diffraction quality single crystals were obtained from a saturated dichloromethane solution at −35 °C. Yield: 81.6 mg (50% isolated yield). 1H NMR (400 MHz, CDCl3): δ 7.81–7.45 (ov, Ar), 4.0 (br, B–H). 19F NMR (377 MHz, CDCl3): δ −135.4 (d, 3JFF = 24.3 Hz, o-C6F5), −135.7 (d, 3JFF = 23.7 Hz, o-C6F5), −158.0 (t, 3JFF = 20.3 Hz, p-C6F5), −158.1 (t, 3JFF = 20.3 Hz, p-C6F5), −164.1 (m, m-C6F5), −164.3 (m, m-C6F5). 11B NMR (128 MHz, CDCl3): δ 0.3 (br). 31P NMR (162 MHz, CDCl3, 298 K): δ 40.8, 37.6 (1: 0.44). 13C{1H} NMR (126 MHz, CDCl3): δ 133.4 (ov), 133.3, 132.3, 132.3, 132.0, 131.9, 131.5, 131.4, 129.1, 129.0, 128.89, 128.86, 128.8, 128.4, 127.2. Elemental analysis: Calc.: C 54.59%, H 2.80%; Exp.: C 54.01%, H 2.64%.
0.5. Diffraction quality single crystals were obtained from a saturated dichloromethane solution at −35 °C. Yield: 81.6 mg (50% isolated yield). 1H NMR (400 MHz, CDCl3): δ 7.81–7.45 (ov, Ar), 4.0 (br, B–H). 19F NMR (377 MHz, CDCl3): δ −135.4 (d, 3JFF = 24.3 Hz, o-C6F5), −135.7 (d, 3JFF = 23.7 Hz, o-C6F5), −158.0 (t, 3JFF = 20.3 Hz, p-C6F5), −158.1 (t, 3JFF = 20.3 Hz, p-C6F5), −164.1 (m, m-C6F5), −164.3 (m, m-C6F5). 11B NMR (128 MHz, CDCl3): δ 0.3 (br). 31P NMR (162 MHz, CDCl3, 298 K): δ 40.8, 37.6 (1: 0.44). 13C{1H} NMR (126 MHz, CDCl3): δ 133.4 (ov), 133.3, 132.3, 132.3, 132.0, 131.9, 131.5, 131.4, 129.1, 129.0, 128.89, 128.86, 128.8, 128.4, 127.2. Elemental analysis: Calc.: C 54.59%, H 2.80%; Exp.: C 54.01%, H 2.64%.
Computational details
Electronic structure calculations were performed using Gaussian 16.33 Geometry optimisations, frequency calculations, and energy determinations were performed using the M062X34 functional and the def2-TZVPP35 basis set with the D3 version of Grimme's dispersion (GD3)36,37 and the dichloromethane solvation effect calculated using the polarisable continuum model (PCM). The absence of any imaginary frequency with an absolute magnitude greater than 10 cm−1 confirmed that each optimised structure was indeed located at a minimum on its potential energy hypersurface. The Gibbs energy corrections from frequency calculations were added to the single-point energies to obtain the Gibbs free energies in solution. Natural bond orbital and natural population analyses were performed on the optimized structures using the M062X functional and def2-TZVP35 basis set using NBO 6.0.38 This work was made possible by the facilities of the Shared Hierarchical Academic Research Computing Network (SHARCNET: http://www.sharcnet.ca) and Compute Canada.Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. W. S. gratefully acknowledges the financial support from the NSERC, Canada, and the award of Canada Research Chair. D. W. S. is also grateful for the award of an Einstein Fellowship at TU Berlin. RLM is grateful for the award of an EPSRC fellowship (CEP R026912/1).Notes and references
- R. G. Hayter, A. W. Laubengayer and P. G. Thompson, J. Chem. Soc., 1957, 4243–4244 CrossRef CAS.
- W. Gerrard, M. F. Lappert and R. Shafferman, J. Chem. Soc., 1958, 3648–3652 RSC.
- A. D. Negro, L. Ungarett and A. Perotti, J. Chem. Soc., Dalton Trans., 1972, 1639–1643 RSC.
- A. Sporzyński, J. Organomet. Chem., 1993, 448, C1–C2 CrossRef.
- R. Köster, A. Sporzyński, W. Schuessler, D. Blaeser and R. Boese, Chem. Ber., 1994, 127, 1191–1199 CrossRef.
- B. Wrackmeyer, E. Khan and R. Kempe, Z. Naturforsch., B: Chem. Sci., 2008, 63, 275–279 CAS.
- M. Periasamy, M. N. Reddy and N. S. Kumar, Sci. Synth., 2005, 6, 321–335 Search PubMed.
- P. Starkov and T. D. Sheppard, Org. Biomol. Chem., 2011, 9, 1320–1323 RSC.
- R. M. Lanigan, P. Starkov and T. D. Sheppard, J. Org. Chem., 2013, 78, 4512–4523 CrossRef CAS PubMed.
- V. Karaluka, R. M. Lanigan, P. M. Murray, M. Badland and T. D. Sheppard, Org. Biomol. Chem., 2015, 13, 10888–10894 RSC.
- P. W. Tang, Org. Synth., 2005, 81, 262–272 CrossRef CAS.
- K. Ishihara, S. Ohara and H. Yamamoto, J. Org. Chem., 1996, 61, 4196–4197 CrossRef CAS PubMed.
- K. Ishihara, S. Ohara and H. Yamamoto, Macromolecules, 2000, 33, 3511–3513 CrossRef CAS.
- T. Maki, K. Ishihara and H. Yamamoto, Synlett, 2004, 1355–1358 CAS.
- A. Sakakura, T. Ohkubo, R. Yamashita, M. Akakura and K. Ishihara, Org. Lett., 2011, 13, 892–895 CrossRef CAS PubMed.
- R. Yamashita, A. Sakakura and K. Ishihara, Org. Lett., 2013, 15, 3654–3657 CrossRef CAS PubMed.
- K. Ishihara and Y. H. Lu, Chem. Sci., 2016, 7, 1276–1280 RSC.
- K. Arnold, B. Davies, D. Herault and A. Whiting, Angew. Chem., Int. Ed., 2008, 47, 2673–2676 CrossRef CAS PubMed.
- K. Arnold, A. S. Batsanov, B. Davies and A. Whiting, Green Chem., 2008, 10, 124–134 RSC.
- R. M. Al-Zoubi, O. Marion and D. G. Hall, Angew. Chem., Int. Ed., 2008, 47, 2876–2879 CrossRef CAS PubMed.
- N. Gernigon, R. M. Al-Zoubi and D. G. Hall, J. Org. Chem., 2012, 77, 8386–8400 CrossRef CAS PubMed.
- T. M. El Dine, J. Rouden and J. Blanchet, Chem. Commun., 2015, 51, 16084–16087 RSC.
- S. Arkhipenko, M. T. Sabatini, A. S. Batsanov, V. Karaluka, T. D. Sheppard, H. S. Rzepa and A. Whiting, Chem. Sci., 2018, 9, 1058–1072 RSC.
- K. Wang, Y. Lu and K. Ishihara, Chem. Commun., 2018, 54, 5410–5413 RSC.
- N. M. Yoon, C. S. Pak, H. C. Brown, S. Krishnam and T. P. Stocky, J. Org. Chem., 1973, 38, 2786–2792 CrossRef CAS.
- H. C. Brown and T. P. Stocky, J. Am. Chem. Soc., 1977, 99, 8218–8226 CrossRef CAS.
- L. L. Liu, L. L. Cao, Y. Shao and D. W. Stephan, J. Am. Chem. Soc., 2017, 139, 10062–10071 CrossRef CAS PubMed.
- L. L. Cao and D. W. Stephan, Organometallics, 2017, 36, 3163–3170 CrossRef CAS.
- A. M. Fuller, D. L. Hughes, S. J. Lancaster and C. M. White, Organometallics, 2010, 29, 2194–2197 CrossRef CAS.
- D. J. Parks, R. E. v. H. Spence and W. E. Piers, Angew. Chem., Int. Ed. Engl., 1995, 34, 809–811 CrossRef CAS.
- I. Justyniak, D. Prochowicz, A. Tulewicz, W. Bury, P. Gos and J. Lewinski, Dalton Trans., 2017, 46, 669–677 RSC.
- R. Kather, E. Rychagova, P. Sanz Camacho, S. E. Ashbrook, J. D. Woollins, L. Robben, E. Lork, S. Ketkov and J. Beckmann, Chem. Commun., 2016, 52, 10992–10995 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, M. C. X. Li, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 09, Revision E.01, Wallingford CT, 2016 Search PubMed.
- Y. Zhao and D. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- S. Grimme, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- E. D. Glendening, J. K. Badenhoop, A. E. Reed, J. E. Carpenter, J. A. Bohmann, C. M. Morales, C. R. Landis and F. Weinhold, NBO 6.0, Madison, WI, 2013 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic and spectral details. CCDC 1883339–1883344. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8dt04818e |
| This journal is © The Royal Society of Chemistry 2019 |