Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Microwave-assisted synthesis: from a mononuclear {CoII} complex to {CoII9} solvomorphs†
Alexandra
Collet
 ,
Claire
Wilson
,
Claire
Wilson
 and
Mark
Murrie
and
Mark
Murrie
 *
*
WestCHEM, School of Chemistry, University of Glasgow, University Avenue, Glasgow G12 8QQ, UK. E-mail: mark.murrie@glasgow.ac.uk
First published on 12th December 2018
Abstract
We report a new {CoII9} complex with an unprecedented structure and its solvated analogue, using microwave heating to tune the synthesis and improve the product selectivity.
Polymetallic 3d and/or 4f complexes have attracted a great deal of interest due to their interesting properties and applications in scientific fields such as molecule-based magnets, magnetic refrigerants, water oxidation electrocatalysts and MRI contrast agents.1 Therefore, the need to synthesise and characterise these complexes continues to grow. Microwave-assisted synthesis is being employed more frequently in inorganic synthesis, instead of conventional heating in an oven, due to three main advantages: reduced reaction times, increased yields and product selectivity.2 However, there are still only limited examples of polynuclear coordination complexes synthesised using this method.3,4
We have investigated the coordination chemistry of the polydentate ligand bicine (H3bic, N,N-bis(2-hydroxyethyl)glycine)5 with CoII. Herein, we report a new, microwave-assisted synthetic procedure for the previously reported [CoII(H2bic)Cl] (1)6 (Fig. 1 left). Furthermore, we present a new nonanuclear complex [CoII9(Hbic)4(bic)2Cl4] (2), with an unprecedented metallic core (Fig. 1 right), and its solvated analogue [CoII9(Hbic)4(bic)2Cl4]·12H2O (2·12H2O), by adjusting the synthetic procedure used for 1. The reaction of CoCl2·6H2O with bicine and NEt3 in EtOH under solvothermal conditions, resulted in a mixture of the complexes 1 (pink block-like crystals), 2 (blue block-like crystals) and 2·12H2O (blue needle-like crystals). However, by using microwave-assisted heating the selectivity is improved (see Scheme 1) and 1, 2 and 2·12H2O can be isolated separately. The full experimental procedure is included in the ESI.†
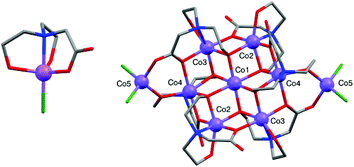 | ||
| Fig. 1 The molecular structure of 1 (left) and [CoII9(Hbic)4(bic)2Cl4] (right). Colour code: CoII: violet, Cl: green, O: red, N: blue, C: grey. Hydrogen atoms are omitted for clarity. | ||
Complex 1 crystallises in the orthorhombic Pbca space group,6 while single-crystal X-ray diffraction for 2 and 2·12H2O revealed that the complexes crystallise in the monoclinic P21/n and trigonal R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space groups, respectively. The crystallographic data for 2 and 2·12H2O can be found in Table S1 (see ESI†). The CoII centre in complex 1 is five-coordinate and adopts a distorted trigonal bipyramidal (TBP) geometry, with one singly deprotonated bicine ligand and one terminal Cl− ligand. Continuous shape measures (CShMs),7 which provide an estimate of the distortion from the ideal TBP geometry for the CoII, give a value of 1.42 (where 0 corresponds to the ideal polyhedron), confirming a significant distortion (Table S2 and Fig. S1, ESI†). Bond Valence Sum (BVS) analysis was used to confirm the oxidation state of CoII.8 Intermolecular hydrogen bonds occur between the hydroxyl groups and the carboxylate groups of neighbouring molecules, forming a two-dimensional network (Fig. S2, ESI†). The shortest intermolecular Co⋯Co′ distances are ∼4.8 Å.6
space groups, respectively. The crystallographic data for 2 and 2·12H2O can be found in Table S1 (see ESI†). The CoII centre in complex 1 is five-coordinate and adopts a distorted trigonal bipyramidal (TBP) geometry, with one singly deprotonated bicine ligand and one terminal Cl− ligand. Continuous shape measures (CShMs),7 which provide an estimate of the distortion from the ideal TBP geometry for the CoII, give a value of 1.42 (where 0 corresponds to the ideal polyhedron), confirming a significant distortion (Table S2 and Fig. S1, ESI†). Bond Valence Sum (BVS) analysis was used to confirm the oxidation state of CoII.8 Intermolecular hydrogen bonds occur between the hydroxyl groups and the carboxylate groups of neighbouring molecules, forming a two-dimensional network (Fig. S2, ESI†). The shortest intermolecular Co⋯Co′ distances are ∼4.8 Å.6
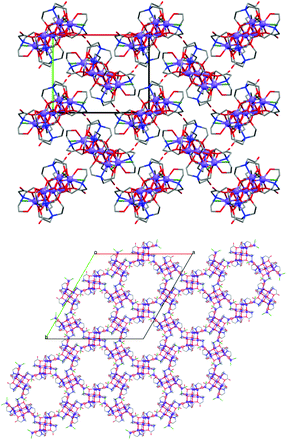
The new nonanuclear complex 2 adopts an unprecedented structural motif which consists of a {CoII7} disk-like core with two additional tetrahedral CoII centres. There are only a few examples of {CoII9} complexes and none of them adopt a similar topology as the complex presented here.9,10 The crystal packing of 2 and 2·12H2O is different (Fig. 2) due to the co-crystallised molecules of water. Small differences are observed in the distortion of the geometries of the cobalt centres and CShMs values for all CoII centres for 2 and 2·12H2O were calculated with the programme SHAPE11 (Tables S3 and S4, ESI†). Seven CoII centres adopt distorted octahedral geometries, while the remaining two adopt slightly distorted tetrahedral geometries. All nine cobalt centres in 2 are in the +2 oxidation state, as confirmed by BVS analysis.8 Five CoII (Co1, Co2, Co2′, Co3, Co3′) centres define a plane while Co4, Co4′, Co5 and Co5′ are located outside this plane (Fig. S3†). This puckered {CoII7} motif is also found in {CoII7} with similar donor atoms and is not likely to be a result of the extra Co5/Co5′ centres.12 Intramolecular interactions are present between the hydroxyl and carboxylate groups of 2 and 2·12H2O. In the solvomorph 2·12H2O intermolecular interactions occur between the hydroxyl and carboxylate groups of neighbouring molecules with the molecules of solvent (see Fig. S4†).
In order to examine if it is possible to isolate the three compounds separately we investigated further by making various changes to the synthetic procedure (e.g. the amount of base, the cobalt salt, the reaction time and/or temperature used in the solvothermal reactions). However, solvothermal conditions always led to a mixture of crystals or crystalline precipitates; therefore, we investigated microwave heating instead. Under microwave heating, in EtOH with a ligand to base ratio ∼4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 a pink crystalline powder is isolated, whereas when the ligand to base ratio is changed to 1
1 a pink crystalline powder is isolated, whereas when the ligand to base ratio is changed to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a blue crystalline powder is formed (for full experimental description see ESI†). Both products were collected by filtration and dried in air, and powder X-ray diffraction (Fig. 3) revealed that the pink and blue products corresponded to complexes 1 and 2·12H2O, respectively. Moreover, when the blue crystalline product is instead collected by filtration and dried under a nitrogen atmosphere, complex 2 is obtained, as confirmed by powder X-ray diffraction (Fig. 3). Hence, the {CoII9} crystal packing (Fig. 2) depends on the drying process (Scheme 1) i.e. drying under an inert atmosphere (glove bag) gives 2, whereas drying in air gives 2·12H2O. Therefore, depending on which drying process is applied, we are able to control which {CoII9} solvomorph is formed.
1, a blue crystalline powder is formed (for full experimental description see ESI†). Both products were collected by filtration and dried in air, and powder X-ray diffraction (Fig. 3) revealed that the pink and blue products corresponded to complexes 1 and 2·12H2O, respectively. Moreover, when the blue crystalline product is instead collected by filtration and dried under a nitrogen atmosphere, complex 2 is obtained, as confirmed by powder X-ray diffraction (Fig. 3). Hence, the {CoII9} crystal packing (Fig. 2) depends on the drying process (Scheme 1) i.e. drying under an inert atmosphere (glove bag) gives 2, whereas drying in air gives 2·12H2O. Therefore, depending on which drying process is applied, we are able to control which {CoII9} solvomorph is formed.
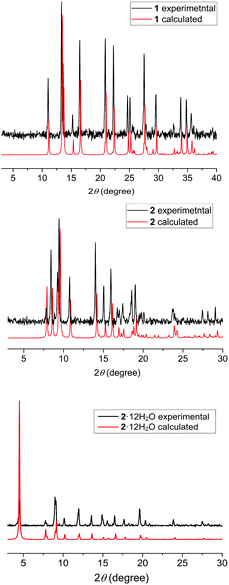 | ||
| Fig. 3 The powder X-ray diffraction patterns of 1 (3–40°), 2 (3–30°) and 2·12H2O (3–30°). The red lines represent the calculated powder X-ray diffraction pattern for each complex and the black lines the experimental ones. All the experimental PXRD patterns were measured at ambient temperature, while the calculated patterns are generated from the single-crystal data collected at 296 K for 1,6 and 100 K for 2 and 2·12H2O. | ||
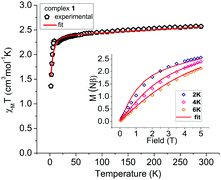
Variable temperature dc susceptibility data were collected for 1, 2 and 2·12H2O in a field of 1000 Oe in the 290–2 K temperature range (Fig. 4 and 5). At 290 K the χMT value of 1 is 2.57 cm3 mol−1 K, higher than the expected χMT = 1.88 cm3 mol−1 K for a high-spin CoII (S = 3/2 and g = 2), indicating a spin–orbit coupling contribution. Upon cooling, χMT decreases slowly until ∼12 K, then increases slightly at 8 K (2.27 cm3 mol−1 K) and drops again to a minimum at 2 K. This behaviour indicates the presence of magnetic anisotropy and weak ferromagnetic intermolecular interactions13 (hydrogen bonds are present and the CoII centres of neighbouring molecules in 1 are close ∼4.8 Å), although we were unable to obtain a satisfactory ferromagnetic zJ term when included in the fit. Magnetisation versus field plots at 2, 4 and 6 K did not saturate, a further indication of the presence of magnetic anisotropy (Fig. 4 inset). The dc magnetic susceptibility data and the magnetisation curves of 1 were fitted simultaneously using the programme PHI14 (Fig. 4). Attempts to fit the data with an anisotropic g value were not successful, while using a positive D did not produce reasonable results, which is a strong indication that an easy-axis magnetic anisotropy is present here. This assumption is reasonable, since the use of a tripodal ligand can enforce C3 symmetry and, as has been seen in previously reported examples, an easy-axis anisotropy (D < 0) can be promoted.15 The extracted parameters from the fitting of the data are: g = 2.25, D = −5.92 (±0.24) cm−1 and E = −1.32 (±0.09) cm−1, with χTIP = 0.0006 cm3 mol−1 for a CoII in TBP geometry.16
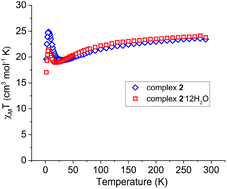 | ||
| Fig. 5 Variable temperature dc susceptibility data for 2 (blue) and 2·12H2O (red) in a field of 1000 Oe from 290–2 K. | ||
The χMT values at room temperature for 2 and 2·12H2O are 23.4 and 23.8 cm3 mol−1 K, respectively, which are higher than the theoretical spin-only value χMT = 16.9 cm3 mol−1 K for nine non-interacting high-spin CoII (S = 3/2 and g = 2), indicating a spin–orbit coupling contribution. χMT slowly decreases with the decrease of temperature until 26 and 19 K for 2 and 2·12H2O, due to the presence of spin–orbit coupling; then χMT increases sharply at 6 K for both complexes reaching the values of 26.2 and 21.5 cm3 mol−1 K for 2 and 2·12H2O respectively, which can be attributed to the presence of ferromagnetic exchange interactions. Finally χMT decreases for both complexes at low temperature, due to zero-field splitting (ZFS) and/or intermolecular antiferromagnetic interactions.
Magnetisation versus field plots at 2, 4 and 6 K did not saturate for both complexes, an indication of the presence of magnetic anisotropy (Fig. S5†). This behaviour is in agreement with other polynuclear CoII-based complexes.4,10,17 It was not possible to fit the data due to the complexity of the system and the presence of octahedral Co(II).
Ac susceptibility measurements were performed for all complexes in order to examine if there is slow relaxation of the magnetisation. Complex 1 does not display any out-of-phase ac signal in zero or an applied dc field. This could be attributed to the significant distortion of the TBP geometry around CoII, the presence of hydrogen bonds between neighbouring molecules and/or the short intermolecular Co⋯Co′ distances (∼4.8 Å). Complex 2 displays only the onset of out-of-phase signals in zero or an applied 2000 Oe dc field (Fig. S6†), while complex 2·12H2O does not display any out-of-phase signals in zero or an applied dc field (Fig. S7, ESI†). The different behaviour between the two {CoII9} complexes could be ascribed to the subtle structural differences between the two complexes (slightly different distortion of the CoII centres, Tables S3 and S4, ESI†) and/or the absence of solvent molecules in the crystal lattice of 2. It has been previously reported that the absence or change of solvent can drastically affect the magnetic properties of a complex.18
In conclusion, we report a new {CoII9} complex [CoII9(Hbic)4(bic)2Cl4] (2), which extends the well-known {CoII7} disk-like structure12,19 with two adjacent tetrahedral CoII centres, and its solvomorph [CoII9(Hbic)4(bic)2Cl4]·12H2O (2·12H2O), and a new synthesis for [CoII(H2bic)Cl] (1).6 In particular, this work highlights the potential of microwave-assisted synthesis in the synthesis of polymetallic complexes using polydentate ligands where bench or solvothermal synthesis leads to a mixture of products. We believe that this approach will prove applicable to a wide range of systems.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the University of Glasgow for funding and the EPSRC UK National Crystallography Service at the University of Southampton for the collection of the crystallographic data.20Notes and references
- J. Ferrando-Soria, J. Vallejo, M. Castellano, J. Martínez-Lillo, E. Pardo, J. Cano, I. Castro, F. Lloret, R. Ruiz-García and M. Julve, Coord. Chem. Rev., 2017, 339, 17–103 CrossRef CAS; Y.-Z. Zheng, G.-J. Zhou, Z. Zheng and R. E. P. Winpenny, Chem. Soc. Rev., 2014, 43, 1462–1475 RSC; R. Brimblecombe, G. F. Swiegers, G. C. Dismukes and L. Spiccia, Angew. Chem., Int. Ed., 2008, 47, 7335–7338 CrossRef PubMed; G. Maayan, N. Gluz and G. Christou, Nat. Catal., 2018, 1, 48–54 CrossRef; G. Guthausen, J. R. Machado, B. Luy, A. Baniodeh, A. K. Powell, S. Krämer, F. Ranzinger, M. P. Herrling, S. Lackner and H. Horn, Dalton Trans., 2015, 44, 5032–5040 RSC; M. Murrie, Polyhedron, 2018, 150, 1–9 CrossRef.
- J. Zhao and W. Yan, in Modern Inorganic Synthetic Chemistry, ed. R. Xu, W. Pang and Q. Huo, Elsevier, Amsterdam, 2011, pp. 173–195 Search PubMed.
- C. J. Milios, A. Prescimone, J. Sanchez-Benitez, S. Parsons, M. Murrie and E. K. Brechin, Inorg. Chem., 2006, 45, 7053–7055 CrossRef CAS PubMed; C. J. Milios, A. Vinslava, A. G. Whittaker, S. Parsons, W. Wernsdorfer, G. Christou, S. P. Perlepes and E. K. Brechin, Inorg. Chem., 2006, 45, 5272–5274 CrossRef PubMed; M.-H. Zeng, Y.-L. Zhou, W.-X. Zhang, M. Du and H.-L. Sun, Cryst. Growth Des., 2010, 10, 20–24 CrossRef; Y.-L. Zhou, M.-H. Zeng, L.-Q. Wei, B.-W. Li and M. Kurmoo, Chem. Mater., 2010, 22, 4295–4303 CrossRef; L.-Q. Wei, K. Zhang, Y.-C. Feng, Y.-H. Wang, M.-H. Zeng and M. Kurmoo, Inorg. Chem., 2011, 50, 7274–7283 CrossRef PubMed; K. Zhang, J. Dai, Y.-H. Wang, M.-H. Zeng and M. Kurmoo, Dalton Trans., 2013, 42, 5439–5446 RSC; H.-G. Jin, X. Jiang, I. A. Kühne, S. Clair, V. Monnier, C. Chendo, G. Novitchi, A. K. Powell, K. M. Kadish and T. S. Balaban, Inorg. Chem., 2017, 56, 4864–4873 CrossRef PubMed.
- L.-Q. Wei, B.-W. Li, S. Hu and M.-H. Zeng, CrystEngComm, 2011, 13, 510–516 RSC.
- K. Graham, A. Darwish, A. Ferguson, S. Parsons and M. Murrie, Polyhedron, 2009, 28, 1830–1833 CrossRef CAS.
- Y. Zhou, X. Liu, Q. Wang, L. Wang and B. Song, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2016, 72, 1463–1467 CrossRef CAS.
- M. Pinsky and D. Avnir, Inorg. Chem., 1998, 37, 5575–5582 CrossRef CAS PubMed; S. Alvarez and M. Llunell, J. Chem. Soc., Dalton Trans., 2000, 3288–3303 RSC.
- N. E. Brese and M. O'Keeffe, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 192–197 CrossRef.
- A. Tsohos, S. Dionyssopoulou, C. P. Raptopoulou, A. Terzis, E. G. Bakalbassis and S. P. Perlepes, Angew. Chem., Int. Ed., 1999, 38, 983–985 CrossRef CAS PubMed; L. N. Dawe, K. V. Shuvaev and L. K. Thompson, Inorg. Chem., 2009, 48, 3323–3341 CrossRef PubMed; E. Fursova, O. Kuznetsova, V. Ovcharenko, G. Romanenko, V. Ikorskii, I. Eremenko and A. Sidorov, Polyhedron, 2007, 26, 2079–2088 CrossRef; S. K. Langley, M. Helliwell, S. J. Teat and R. E. P. Winpenny, Dalton Trans., 2012, 41, 12807–12817 RSC; W. Shentang, B. Yanfeng, H. Xinxin, Z. Xiaofei and L. Wuping, Z. Anorg. Allg. Chem., 2017, 643, 160–165 CrossRef.
- G. S. Papaefstathiou, A. K. Boudalis, T. C. Stamatatos, C. J. Milios, C. G. Efthymiou, C. P. Raptopoulou, A. Terzis, V. Psycharis, Y. Sanakis, R. Vicente, A. Escuer, J.-P. Tuchagues and S. P. Perlepes, Polyhedron, 2007, 26, 2089–2094 CrossRef CAS.
- S. Alvarez, D. Avnir, M. Llunell and M. Pinsky, New J. Chem., 2002, 26, 996–1009 RSC; C. Jordi, A. Pere and A. Santiago, Chem. – Eur. J., 2004, 10, 190–207 CrossRef CAS PubMed.
- R. Pattacini, P. Teo, J. Zhang, Y. Lan, A. K. Powell, J. Nehrkorn, O. Waldmann, T. S. A. Hor and P. Braunstein, Dalton Trans., 2011, 40, 10526–10534 RSC; S.-H. Zhang, L.-F. Ma, H.-H. Zou, Y. G. Wang, H. Liang and M. H. Zeng, Dalton Trans., 2011, 40, 11402–11409 RSC; R. Modak, Y. Sikdar, A. E. Thuijs, G. Christou and S. Goswami, Inorg. Chem., 2016, 55, 10192–10202 CrossRef CAS PubMed.
- Y.-Z. Zhang, W. Wernsdorfer, F. Pan, Z.-M. Wang and S. Gao, Chem. Commun., 2006, 3302–3304 RSC; T. Jurca, A. Farghal, P.-H. Lin, I. Korobkov, M. Murugesu and D. S. Richeson, J. Am. Chem. Soc., 2011, 133, 15814–15817 CrossRef CAS PubMed; F. Habib, O. R. Luca, V. Vieru, M. Shiddiq, I. Korobkov, S. I. Gorelsky, M. K. Takase, L. F. Chibotaru, S. Hill, R. H. Crabtree and M. Murugesu, Angew. Chem., Int. Ed., 2013, 52, 11290–11293 CrossRef PubMed.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, J. Comput. Chem., 2013, 34, 1164–1175 CrossRef CAS PubMed.
- I. Nemec, R. Marx, R. Herchel, P. Neugebauer, J. van Slageren and Z. Trávníček, Dalton Trans., 2015, 44, 15014–15021 RSC; R. Ruamps, L. J. Batchelor, R. Guillot, G. Zakhia, A.-L. Barra, W. Wernsdorfer, N. Guihéry and T. Mallah, Chem. Sci., 2014, 5, 3418–3424 RSC; D. Schweinfurth, J. Krzystek, M. Atanasov, J. Klein, S. Hohloch, J. Telser, S. Demeshko, F. Meyer, F. Neese and B. Sarkar, Inorg. Chem., 2017, 56, 5253–5265 CrossRef CAS PubMed; T. J. Woods, M. F. Ballesteros-Rivas, S. Gómez-Coca, E. Ruiz and K. R. Dunbar, J. Am. Chem. Soc., 2016, 138, 16407–16416 CrossRef PubMed; S. Tripathi, A. Dey, M. Shanmugam, R. S. Narayanan and V. Chandrsekhar, in Topics in Organometallic Chemistry, Springer, Berlin, Heidelberg, 2018, pp. 1–41 Search PubMed.
- R. Herchel and R. Boča, Dalton Trans., 2005, 1352–1353 RSC; J. S. Wood, J. Chem. Soc. A, 1969, 1582–1586 RSC.
- X.-T. Wang, B.-W. Wang, Z.-M. Wang, W. Zhang and S. Gao, Inorg. Chim. Acta, 2008, 361, 3895–3902 CrossRef CAS; S.-H. Zhang, R.-X. Zhao, G. Li, H.-Y. Zhang, C.-L. Zhang and G. Muller, RSC Adv., 2014, 4, 54837–54846 RSC; T. Singha Mahapatra, D. Basak, S. Chand, J. Lengyel, M. Shatruk, V. Bertolasi and D. Ray, Dalton Trans., 2016, 45, 13576–13589 RSC.
- J.-Y. Ge, L. Cui, J. Li, F. Yu, Y. Song, Y.-Q. Zhang, J.-L. Zuo and M. Kurmoo, Inorg. Chem., 2017, 56, 336–343 CrossRef CAS PubMed; C.-M. Liu, D.-Q. Zhang and D.-B. Zhu, Sci. Rep., 2017, 7, 15483 CrossRef PubMed; W.-Y. Zhang, Y.-Q. Zhang, S.-D. Jiang, W.-B. Sun, H.-F. Li, B.-W. Wang, P. Chen, P.-F. Yan and S. Gao, Inorg. Chem. Front., 2018, 5, 1575–1586 RSC.
- A. B. Canaj, L. E. Nodaraki, K. Ślepokura, M. Siczek, D. I. Tzimopoulos, T. Lis and C. J. Milios, RSC Adv., 2014, 4, 23068–23077 RSC.
- S. J. Coles and P. A. Gale, Chem. Sci., 2012, 3, 683–689 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1878568–1878569. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8dt04557g |
| This journal is © The Royal Society of Chemistry 2019 |