Amorphous Fe2O3 for photocatalytic hydrogen evolution†
Abstract
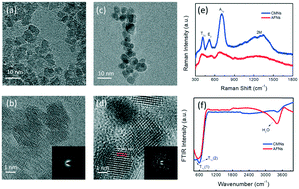
Fe2O3 has drawn significant attention in photocatalysis due to its natural abundance, thermodynamic stability, environmental compatibility, low toxicity and narrow bandgap. Here, for the first time, we demonstrate that amorphous Fe2O3 nanoparticles can act as efficient and robust photocatalysts for solar H2 evolution without any cocatalysts. We also establish a plausible mechanism involving the amorphization-induced thermodynamic and dynamic behaviors of amorphous Fe2O3 upon photocatalytic hydrogen evolution. Thermodynamically, amorphization provides more surface states and larger carrier density, and thus elevates the conduction band edge to go across the H2 evolution potential level. Dynamically, amorphization-induced crystal field splitting weakening delocalizes the photogenerated carriers, and thus overcomes the excitation-wavelength-dependent small polaron trapping effect. These findings imply that amorphization may be a promising approach to functionalize and tailor other photocatalysts.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...