Characteristics of l-threonine transaldolase for asymmetric synthesis of β-hydroxy-α-amino acids†
Abstract
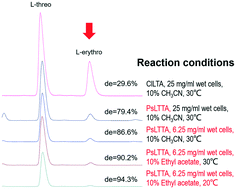
L-Threonine transaldolase (LTTA) is a putative serine hydroxymethyltransferase (SHMT) that can catalyze the trans-aldehyde reaction of L-threonine and aldehyde to produce L-threo-β-hydroxy-α-amino acids with excellent stereoselectivity. In the present study, an L-threonine transaldolase from Pseudomonas sp. (PsLTTA) was mined and expressed in Escherichia coli BL21 (DE3). A substrate spectrum assay indicated that PsLTTA only consumed L-threonine as the donor substrate and could accept a wide range of aromatic aldehydes as acceptor substrates. Among these substrates, PsLTTA could catalyze p-methylsulfonyl benzaldehyde and L-threonine to produce L-threo-p-methylsulfonylphenylserine with a high conversion rate (74.4%) and a high de value (79.9%). The conversion and stereoselectivity of PsLTTA were found to be dramatically influenced by the concentration of the whole cell, the co-solvent and the reaction temperature. Through conditional optimization, L-threo-p-methylsulfonylphenylserine was obtained with 67.1% conversion and a near-perfect de value (94.5%), the highest stereoselectivity for an L-threo-β-hydroxy-α-amino acid so far reported by enzymatic synthesis. Finally, synthesis of L-threo-p-methylsulfonylphenylserine at a 100 mL scale by whole-cell biocatalysis was conducted. This is the first systematic report of L-threonine transaldolase as a robust biocatalyst for preparation of β-hydroxy-α-amino acids, which can provide new insights for β-hydroxy-α-amino acids synthesis.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...