Chiral salen Cr(iii) complexes encapsulated in thermo-responsive polymer nanoreactors for asymmetric epoxidation of alkenes in water†
Abstract
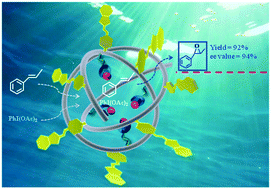
Chiral salen Cr(III) (Cr(salen)) complexes were encapsulated in thermo-responsive polymer nanoreactors through folding an amphiphilic random copolymer of poly(N-isopropylacrylamide-co-IL/Cr(salen)) (poly(NIPAAM-co-IL/Cr(salen)) around Cr(salen) in water. The resulting catalytic nanoreactors exhibited several advantages over the traditional Cr(salen) system for asymmetric epoxidation of alkenes in water. First, they were dispersed in water, behaving as a quasi-homogeneous catalyst for the aqueous asymmetric epoxidation. Second, they effectively sequestered substrates from the surrounding environment, creating a highly concentrated environment for efficient catalysis. Third, water was excluded from the nanoreactor, minimizing the undesired hydrolysis of epoxides. As a result, the compartmentalized catalysts mediated aqueous asymmetric epoxidation with unprecedented yields (92–95%) and enantioselectivities (ee, 92–99%), whereas the traditional Cr(salen) catalyst was far less efficient (4–12% yield and 29–44% ee). Moreover, the catalytic nanoreactor could be facilely recovered for reuse by thermo-controlled separation. This work highlighted the potential of using folded polymers as a platform for developing highly efficient catalytic nanoreactors for a number of important organic transformations in water.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...