Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A step forward in solvent knitting strategies: ruthenium and gold phosphine complex polymerization results in effective heterogenized catalysts†
Antonio
Valverde-González
 ,
Gwendoline
Marchal‡
,
Eva M.
Maya
,
Gwendoline
Marchal‡
,
Eva M.
Maya
 * and
Marta
Iglesias
* and
Marta
Iglesias
 *
*
Instituto de Ciencia de Materiales de Madrid, CSIC, c/Sor Juana Inés de la Cruz 3, Cantoblanco, Madrid 28049, Spain. E-mail: marta.iglesias@icmm.csic.es
First published on 15th July 2019
Abstract
Porous polymers based on ruthenium and gold triphenylphosphine complexes (KPhos(Ru), KPhos(Ru)Bi, KPhos(AuCl) and KPhos(AuNTf2)) were prepared via a cost-effective solvent knitting method with [RuHClCO(PPh3)3] or AuXPPh3 (X = Cl, NTf2) as single monomers or combined with biphenyl, which represents a further approach to obtain heterogenized catalysts. The resulting materials mainly preserve the metal coordination environment of their parent complexes, are stable up to 350 °C and have reasonable surface areas (250–300 m2 g−1 for KPhos(Ru)-polymers). KPhos(Ru)s selectively catalyze the imination of alcohols in the presence of base and the results for KPhos(Au)s show they are effective for the intermolecular hydration and hydroamination of alkynes. These materials can be reused several times without significant loss of activity. This novel and simple method affords heterogenized catalysts that combine the reactivity and selectivity of their homogeneous counterparts with the stability and reusability of a heterogeneous framework.
Introduction
Heterogenized catalysts are usually prepared by immobilizing a homogeneous catalyst onto a solid support and have shown significant benefits in stability, recyclability over multiple cycles, and separation from the reactants.1 Silica-based materials2 and magnetic nanoparticles3 are usually used as supports, but they have the disadvantages of a lack of homogeneity of the active sites and eventual blocking of the pores and deactivation of the catalyst.4 Over the last few years porous hybrid organo–inorganic materials (MOFs),5 and porous organic materials as porous organic polymers (POPs)6 or covalent organic frameworks (COFs)7 have been used as solid porous platforms to heterogenize homogeneous catalysts. In general, POPs show high hydrothermal and chemical stability and could be converted into catalysts by incorporation of catalytic sites as building blocks by covalent bonds and can be modified by post-synthetic methods.8Hyper-cross-linked polymers synthesized by a knitting polymerization method (employing AlCl3 or FeCl3 as a catalyst and dichloromethane or dimethoxymethane as a cross-linker)9 are a relative new family of porous polymers with excellent properties, such as high thermal and chemical stability, insolubility and easy post-functionalization for use as supports for homogeneous catalysts. This method has been applied to prepare several series of porous polymers from different aromatic monomers.6f,10
Nonetheless, the maintenance of the coordination metal environment through this strategy has not always been pursued. Lai and co-workers reported the direct knitting of palladium tetrakis(triphenylphosphine) and benzene where the palladium atoms were spatially isolated in the framework of the polymer.11 Alternatively, Song and co-workers knitted 1,1′-bis(diphenylphosphino)ferrocene and biphenyl, affording an effective catalyst for the reduction of 4-nitrophenol, where the ferrocenyl units were not altered.12 Recently, during the preparation of this manuscript, Huang's group achieved a highly effective catalyst for the dehydrogenation of formic acid to hydrogen by knitting a pincer ruthenium complex and benzene.13
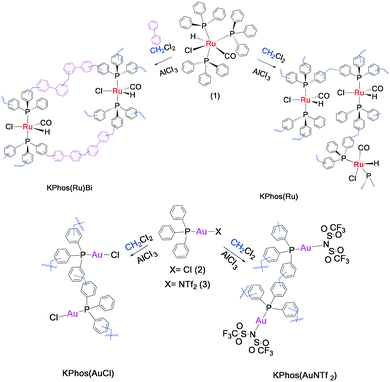
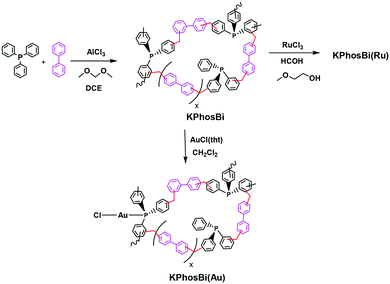
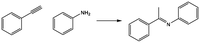
Here, we present an easy synthesis of new knitted metal-based polymers that maintains the molecular structure of the parent Ru- and Au-complexes (Scheme 1); these materials result in effective heterogenized metal-triphenylphosphine catalysts for the synthesis of imines, in the case of the Ru-based polymers, and for the hydration of alkynes, in the case of the Au-based catalysts.
Results and discussion
The knitted ruthenium and gold–phosphine-polymers (KPhos(Ru), KPhos(Ru)Bi, KPhos(AuCl), KPhos(AuNTf2)) were synthesized in a one-step hyper-crosslinking method via an AlCl3-catalyzed reaction (Scheme 1).10 In this method, dichloromethane was employed as an external methylene linker between the phenyl groups from the phosphine ligands in the parent ruthenium [RuHClCO(PPh3)3] or AuXPPh3 (X = Cl, NTf2) complexes. In the case of KPhos(Ru)Bi, the polymerization occurs in the presence of biphenyl, which acts as a co-monomer in a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 versus the ruthenium complex.
1 versus the ruthenium complex.
The resulting polymers are insoluble in water, alcohol or the most common organic solvents. The formation of the hyper-cross-linked metal–phosphine complex network is confirmed by FTIR, solid-state 13C-NMR, 31P-NMR, thermal analysis (TGA), and SEM and TEM microscopies. X-ray photoelectron spectroscopy (XPS) confirms the oxidation state of ruthenium, gold and phosphorous. The metal loading was determined by transmission X-ray fluorescence spectroscopy (TXRF) or ICP-OES14 with the results: 2.26 mmol g−1, 22% weight (KPhos(Ru)), 0.79 mmol g−1, 8% weight (KPhos(Ru)Bi), 0.72 mmol g−1, 15% weight (KPhos(AuNTf2)) and 0.97 mmol g−1, 19% weight (KPhos(AuCl)). SEM-EDX analyses confirmed that the ratios of P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ru and P
Ru and P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au are 2
Au are 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively (Fig. S17†). These results are in good agreement with the inorganic residue obtained by TGA.
1, respectively (Fig. S17†). These results are in good agreement with the inorganic residue obtained by TGA.
The 13C-CP/MAS-NMR spectra of the polymers (Fig. 1) displayed a peak at δ = 41.2 ppm corresponding to methylene linker, from CH2Cl2, which confirms the successful C–C coupling.15 The CO signals could not be identified because of the low sensitivity of solid-state NMR spectroscopy. The peaks at δ ∼ 139 (sh) and 131 ppm correspond to aromatic carbons from triphenylphosphine ligands.16 The 31P-MAS-NMR spectra (Fig. S1 and S2†) show peaks at 28 and 26 ppm for both KPhos(Ru) and KPhos(Ru)Bi polymers, at 29 ppm for KPhos(AuCl) and at 26 ppm for KPhos(AuNTf2) which confirm the presence of P–Ru and P–Au bonds. Further peaks at δ ∼ 60–70 ppm are attributable to phosphorous species generated by the polymerization process (for example ionic phosphonium species such as (PPh3Cl)Cl).17 It is important to note that no free phosphine (at ∼5 ppm) was observed.
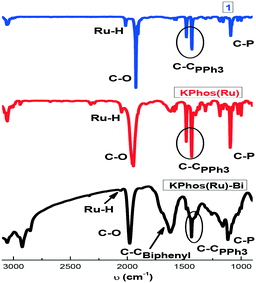
FTIR spectra (Fig. 2) show bands at 2058 and 1980 cm−1 corresponding to ν(Ru–H) and ν(CO), respectively, confirming that the molecular structure is mainly maintained after the polymerization process; some other vibration bands are also present, such as ν(C–H) at 3100 cm−1, ν(C–C) at ∼1400 cm−1 from triphenylphosphine moieties and ν(P–C) at 1100 cm−1. The KPhos(Ru)Bi spectrum also shows an extra band at ∼1600 cm−1 corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration from biphenyl. The FT-IR spectra of the gold-polymers are shown in Fig. S5.†
C vibration from biphenyl. The FT-IR spectra of the gold-polymers are shown in Fig. S5.†
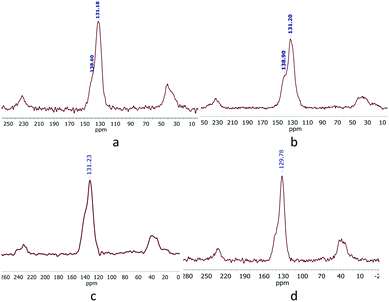
Fig. S16† shows the XPS survey scans of the polymers. Ru was analysed by its 3p state instead of the 3d spectra to avoid the overlap of the C1s and Ru3d core-levels. The Ru3p region (Fig. 3a) shows a doublet peak at 464 eV and 485 eV for Ru3p3/2 and Ru3p1/2, respectively, for the RuP species, which confirms that ruthenium has the +2-oxidation state.18 The P2p spectrum (Fig. 3b) showed the presence of only one P-containing species at 133.0 eV, assigned to the PPh3 species. For KPhos(AuCl), the binding energy of Au4f at 86 eV justifies the presence of the gold(I) oxidation state (Fig. 3c); the typical P2p binding energy was observed at 134 eV, confirming that no triphenylphosphine oxide was present in the sample. These results confirm that the oxidation state of the polymer is preserved.
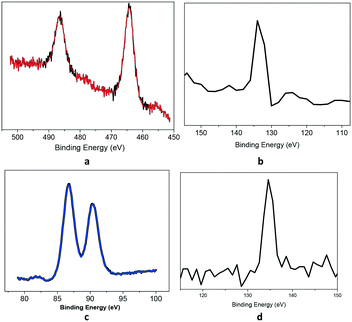 | ||
| Fig. 3 Core-level XPS spectra: (a) Ru(3p) and (b) P(2p) (KPhos(Ru)) (top), and (c) Au(4f) and (d) P(2p) (KPhos(AuCl)) (bottom). | ||
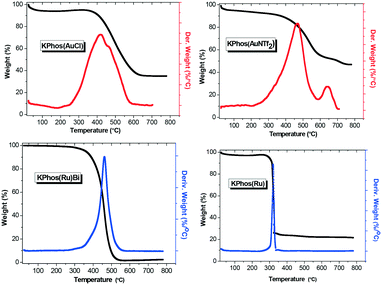
Thermogravimetric analyses (TGA) (Fig. 4) showed a one-step degradation pattern for all the knitted metal–phosphine polymers. KPhos(Ru)Bi exhibited higher thermal stability than KPhos(Ru) with thermal decomposition temperatures of 380 °C and 300 °C, respectively. Whereas knitted gold–phosphine polymers showed similar degradation temperatures around 350 °C. As commented previously, the metal content was confirmed by TGA. At high temperatures, gold, ruthenium and phosphine oxides are formed, Au2O3, RuO2 and P2O5; however, at 200 °C Au2O3 decomposed into Au and O2.19 Therefore, considering that the Ru-polymer residue is RuO2 and P2O5 and Au and P2O5 for the Au-polymers, it was possible to estimate the gold, ruthenium and phosphorous loading by TGA analyses which were in good agreement with those obtained by TXRF and ICP-OES, as given above.
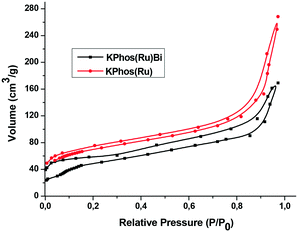
The N2 adsorption isotherms of both KPhos(Ru)Bi and KPhos(Ru) displayed the same type II profile. The Brunauer–Emmett–Teller (BET) surface areas were 250 m2 g−1 and 300 m2 g−1 (Fig. 5, Table 1) and the total pore volumes were found to be 0.11 and 0.20 cm3 g−1, respectively. The pore distributions were calculated by the N2-DFT method (Fig. S13†). The plot shows that pore sizes were mainly distributed around 2.4 nm, indicating that mesopores were present in both polymers. Furthermore KPhos(Ru)Bi also exhibited mesopores around 40 nm. KPhos(AuNTf2) and KPhos(AuCl) polymers showed low N2 adsorption (Fig. S14, A and B†) affording only 20 and 49 m2 g−1 of specific surface areas, respectively.
| Polymer | S BET (m2 g−1) | V TOTAL (cm3 g−1) | D pore (nm) |
|---|---|---|---|
| a At P/P0 = 0.99. | |||
| KPhos(Ru) | 300 | 0.203 | 2.2 |
| KPhos(Ru)Bi | 250 | 0.107 | 2.2 |
| KPhosBi | 704 | 1.45 | 8.3 |
| KPhos(AuNTf 2 ) | 20 | 0.12 | 1.7 |
| KPhos(AuCl) | 49 | 0.07 | 5.9 |
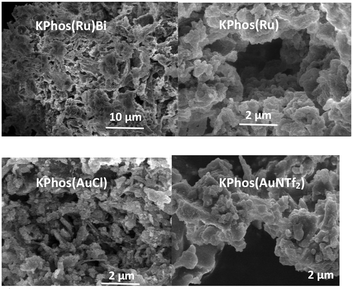
The surface morphology was explored by field-emission scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Fig. 6 and S7–S12†) showing the typical morphology described for this type of porous organic polymer, made up of aggregates of spherical structures that resemble the texture of a sponge. In addition, energy-dispersive X-ray spectroscopy (SEM–EDX) and elemental mapping analysis revealed coherent ratios of Ru/P and Au/P (see the ESI,† Fig. S17).
Post-funtionalization of triphenylphosphine knitted polymers (KPhosBi(M), M: Ru, Au)
For comparative purposes, we have tried to prepare polymers following a post-functionalization strategy (Scheme 2), using a similar approach to that previously reported with benzene as a co-linker.20 Triphenylphosphine was reacted with biphenyl (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the presence of AlCl3 and dimethoxymethane as an external linker in dichloroethane (DCE) as a solvent, yielding a polymer denoted KPhosBi. It showed a BET surface area of 704 m2 g−1 (Table 1) (N2 adsorption/desorption isotherm, Fig. S15†). Its NMR solid state spectra are shown in Fig. S3.† Then, KPhosBi was reacted with RuCl3.nH2O, following a similar method to the one used to synthesize the precursor RuHClCO(PPh3)3. However, TXRF and XPS analyses of the resulting solid revealed that the ruthenium loading in KPhosBi(Ru) is only 0.02 mmol g−1 (Fig. S16†). Therefore, we discarded this approach to heterogenizing the Ru–phosphine complex. Alternatively, KPhosBi was reacted with AuCl(tht) in dichloromethane, affording the corresponding KPhosBi(Au) polymer. ICP-OES analysis revealed that the gold loading was 1.01 mmol g−1. The 31P-NMR spectrum shows that P–Au and phosphine moieties are present in the polymer (Fig. S4†). XPS analysis shows a doublet corresponding to Au4f at around 85 eV. In both spectra, the characteristic phosphine peak of P2p was observed at around 136 eV (Fig. S16†). In this case, it seems that the corresponding supported Au–phosphine complex has been formed.
1) in the presence of AlCl3 and dimethoxymethane as an external linker in dichloroethane (DCE) as a solvent, yielding a polymer denoted KPhosBi. It showed a BET surface area of 704 m2 g−1 (Table 1) (N2 adsorption/desorption isotherm, Fig. S15†). Its NMR solid state spectra are shown in Fig. S3.† Then, KPhosBi was reacted with RuCl3.nH2O, following a similar method to the one used to synthesize the precursor RuHClCO(PPh3)3. However, TXRF and XPS analyses of the resulting solid revealed that the ruthenium loading in KPhosBi(Ru) is only 0.02 mmol g−1 (Fig. S16†). Therefore, we discarded this approach to heterogenizing the Ru–phosphine complex. Alternatively, KPhosBi was reacted with AuCl(tht) in dichloromethane, affording the corresponding KPhosBi(Au) polymer. ICP-OES analysis revealed that the gold loading was 1.01 mmol g−1. The 31P-NMR spectrum shows that P–Au and phosphine moieties are present in the polymer (Fig. S4†). XPS analysis shows a doublet corresponding to Au4f at around 85 eV. In both spectra, the characteristic phosphine peak of P2p was observed at around 136 eV (Fig. S16†). In this case, it seems that the corresponding supported Au–phosphine complex has been formed.
Catalytic applications
It is well known that ruthenium is a widely used and versatile catalyst for the dehydrogenation of alcohols.24 However, it is hardly used for the imination reaction.25 Ru-parent complex 1 is an efficient homogeneous catalyst in reactions such as the hydrogenation of aldehydes and ketones, transfer hydrogenation or transfer hydrogenative C–C bond forming reactions.26 Thus, these data prompted us to study a possible imination reaction from alcohols and amines with knitted Ru-polymers as heterogeneous catalysts and the results were compared with those obtained from a homogeneous 1-complex. Since KPhos(Ru) and KPhos(Ru)Bi have similar characteristics, we chose KPhos(Ru) as the reference catalyst. Reaction of 1.2 mmol of benzyl alcohol, 1.0 mmol of aniline and 2 mol% of catalyst in toluene at 100 °C for 2 h in the presence of a base, quantitatively afforded N-benzylidenebenzylamine (96% selectivity) (Table 2, entry 1). Previous experiments revealed that the reaction in the presence of KOH resulted in longer reaction times (entry 4) than using potassium tert-butoxide (entry 3), to achieve a similar conversion with the same substrate. Moreover, it was tested that the reaction did not occur without a base (entry 5). The reaction was not efficient at room temperature and the best results were obtained in toluene at 100 °C. In the absence of a catalyst, only traces of product were obtained (entry 6).
| Entry | Catalyst | Base | R | t (h) | Conv. (%) | Sel. (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: T: 100 °C, amine (1.0 mmol), benzyl alcohol (1.2 mmol), toluene (0.5 mL), base (0.05 mol%), cat. (2.0% mmol based on Ru). b 0.5% mol. | ||||||
| 1 | KPhos(Ru) | KOtBu | Ph | 2 | 100 | 96 |
| 2 | RuHCl(CO)(PPh 3 ) 3 | KOtBu | Ph | 0.5 | 100 | 98 |
| 3 | KPhos(Ru) | KOtBu | 4-OMe-Ph | 1.5 | 100 | 100 |
| 4 | KPhos(Ru) | KOH | 4-OMe-Ph | 4 | 97 | 100 |
| 5 | KPhos(Ru) | None | Ph | 20 | 0 | — |
| 6 | None | KOtBu | Ph | 6 | 0.8 | 100 |
| 7 | KPhos(Ru) | KOtBu | PhCH2 | 2 | 100 | 100 |
| 8 | KPhos(Ru) | KOtBu | CH3(CH2)7 | 4 | 100 | 100 |
| 9 | KPhos(Ru) | KOtBu | 4-Br–Ph | 20 | 90 | 100 |
The substrate scope was examined under optimized conditions. Electron-rich 4-methoxyaniline, benzylamine and 1-octylamine gave excellent conversions and selectivity into the corresponding imines (Table 2, entries 3, 7, 8), and 4-bromoaniline afforded 90% conversion after 20 h of reaction (entry 9).
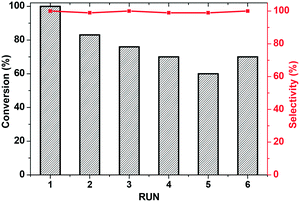
The possibility of recycling and reusing the catalyst, along with the occurrence of leaching processes, were examined under the conditions in entry 1. Recycling experiments show that the activity of KPhos(Ru) does partially decrease after two cycles, probably due to some loss of catalyst during filtration, and more time is needed to achieve >90% conversion (Fig. 7). In order to evaluate whether the structure of the catalyst had changed after recycling, we undertook XPS analysis of the recovered catalyst (Fig. S21†). The XPS spectrum in the region of Ru3p and P2p is very similar to that of fresh Ru-polymer. FT-IR after recycling (see Fig. S6†) shows that ν(Ru–H) appears as weak signal at 2055 cm−1 and ν(CO) at 1982 cm−1 which indicates that the coordination environment of ruthenium in the polymer is mainly maintained. Gratifyingly, no leaching of active ruthenium species occurred. Upon removal of the catalyst by hot filtration after 30 minutes of reaction, the conversion remained unaltered for 3 h (Fig. S18b†).
KPhos(Ru) was also investigated for the transfer hydrogenation of acetophenone in i-PrOH at reflux, resulting in total conversion after 16 h of reaction. The reaction between benzyl alcohol and acetophenone leads to a mixture of condensation products ketone/alcohol (80/20).
Based on our results and previous reports, the proposed mechanism for the alkylation of amine with alcohol27 is that a Ru–alkoxide intermediate is formed under basic conditions, which catalyses the dehydrogenation of alcohol to a carbonyl compound, and subsequently, the base promotes the condensation of carbonyl compound with amine to imine.
First, we carried out reactions with KPhos(AuCl) polymer in the presence of different silver-containing cocatalysts (Table 3, entries 1–3) in order to remove the halide coordinated to the gold(I) centres and generate catalytically suitable species for substrate activation ([PPh3Au]+ species). It was found that the best results were obtained in the presence of silver triflimide because the triflimide anion stabilizes the cation [PPh3Au]+, and in all cases, the ketone was obtained. The reaction evolution in the presence of silver co-catalysts is shown in Fig. S19.† Therefore, the system KPhos(AuCl)/AgNTf2 was employed with different terminal alkynes affording total conversion after 1 h of reaction (entries 11–13). However, when the internal alkyne methyl-phenylacetylene was employed, the reaction did not occur even after 20 hours (entry 14).
| Entry | Catalyst | Alkyne | t (h) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: T: 60 °C, alkyne (0.2 mmol), MeOH (0.4 mL), H2O (20 μL) cat 5 mol% (based on Au), co-catalyst: 5.5 mol%. | ||||
| 11 | KPhos(AuCl)/AgNTf2 |
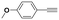
|
1 | 100 |
| 12 |

|
1 | 100 | |
| 13 |

|
1 | 100 | |
| 14 |

|
20 | Traces | |
Some other silver-free co-catalysts were used, such as: CH3CN/NH4BF4, TfOH and LiNTf2(entries 4–6). Again, the best results were obtained in the presence of lithium triflimide (entry 6). However, longer reaction times were necessary to achieve 100% yield. Rather, when the post-functionalized KPhosBi(AuCl)/LiNTf2 system was applied as a catalyst, only traces of hydrated product were detected after 3 hours of reaction, and longer reaction times were necessary to achieve good conversion (entry 7).
The use of silver salts as cocatalysts significantly affected the reusability of the catalyst, since after three cycles the catalyst lost its efficiency. This might be a consequence of the so-called ‘silver effect’, reported by Shi and coworkers,28 which suggests that silver cations not only activate gold moieties but also modify them, affording different inactive complexes. Subsequently, the possibility of recycling the catalyst, along with the occurrence of leaching processes, was examined with the KPhos(AuCl)/LiNTf2 system. Even though the catalyst life has been increased, it can clearly be observed that its activity decreases in each cycle (Fig. 8). Therefore, we tried to regenerate the catalyst by treating it with LiNTf2 in methanol overnight, and we observed that the activity increased slightly up to 70%.
With the previous results in hand, we explored the catalytic activity of KPhos(AuNTf2) under standard conditions (Table 4). It was found that 4-methoxy phenylacetylene and 1-octyne quantitatively yields the ketone after 1 h in the presence of 20 μl of water (entries 1 & 3); when the reaction was performed without water the ketone was not obtained (entries 2 & 4). When the same conditions were applied to internal alkynes, it was found that methyl phenylacetylene led selectively to the ketone after 3 h of reaction with a higher amount of water (entry 6); however, diphenylacetylene only yields the enol ether (Table 3, product A) after 22 h (entries 7, 8) and 1,4-dichlorobut-2-yne does not react (entry 9). These findings suggest the following: (A) the ketone is only formed after hydration of ketal, as was previously reported by Corma et al.29 (B) The ketal of the terminal alkyne undergoes hydration to ketone faster than those of the internal alkynes.
| Entry | Alkyne | H2O (μL) | t (h) | Conv. (%) | Selec. (%) (A/B/C) |
|---|---|---|---|---|---|
| a Reaction conditions: T: 60 °C, alkyne (0.2 mmol), MeOH (0.4 mL), cat (10 mg, 7.2 μmol, 2.8 mol% based on Au). | |||||
| 1 |
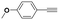
|
20 | 1 | 100 | 0/0/100 |
| 2 | — | 2 | 80 | 90/10/0 | |
| 3 |
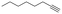
|
20 | 1 | 100 | 0/traces/99 |
| 4 | — | 1 | 75 | 70/30/0 | |
| 5 |
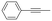
|
20 | 3 | 100 | 15/85/0 |
| 6 | 40 | 3 | 100 | 0/0/100 | |
| 7 |

|
20 | 3 | 30 | 100/0/0 |
| 8 | 22 | 75 | 100/0/0 | ||
| 9 |
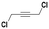
|
20 | 22 | 0 | — |
In order to verify the heterogeneity of the gold-catalyst, a hot filtration experiment was carried out. After 20 minutes, the solid was separated from the reaction media and the filtrate was stirred under the same conditions. Since the conversion did not increase after hot filtration, this experiment confirmed that no leaching occurs during the process (see Fig. S20†).
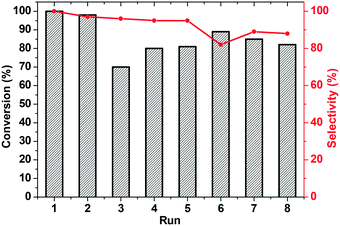
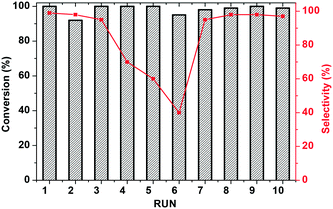
Finally, recycling of KPhos(AuNTf2) using 4-methoxy phenylacetylene as a substrate was performed under entry 1 conditions. As can be observed in Fig. 9, the catalyst maintains its activity for ten cycles; however, the selectivity decreases after the third run and decreases to 40% in the sixth cycle. It is therefore necessary to regenerate the catalyst, which is carried out by stirring it in methanol in the presence of LiNTf2 overnight. This reactivation resulted in an increase in the selectivity to 100% for four more cycles.
| Entry | Catalyst | Co-catalystb | t (h) | Conv. (%) | Selec. (%) |
|---|---|---|---|---|---|
| a Reaction conditions: T: 70 °C, amine (0.17 mmol), alkyne (0.15 mmol), toluene (0.5 mL) and cat. (3.3 mg, 3.2 μmol, 2 mol% based on Au). b Co-catalyst: 2.2 mol%. | |||||
| 1 | KPhos(AuNTf 2 ) | — | 2 | 85 | 98 |
| 2 | KPhos(AuCl) | AgBF4 | 23 | 100 | 98 |
| 3 | KPhos(AuCl) | AgOTf | 2 | 100 | 94 |
| 4 | KPhos(AuCl) | AgNTf2 | 2 | 100 | 100 |
| 5 | KPhos(AuCl) | CH3CN/NH4BF4 | 1.5 | 100 | 97 |
| 6 | KPhos(AuCl) | — | 27 | 53 | 53 |
| 7 | AgBF 4 | — | 24 | 28 | 45 |
Conclusions
This manuscript presents four new heterogenized catalysts formed by the polymerization of phosphine metal complexes via a solvent knitting method. In this way, the polymerization of RuH(CO)Cl(PPh3)3 resulted in a single-site robust material, which maintained its catalytic activity after six cycles in the synthesis of imines. On the other hand, the highly active homogeneous catalyst Au(PPh3)NTf2 was polymerized by the same procedure, leading to a heterogeneous framework that also maintained its catalytic activity even after 10 cycles in the hydration of alkynes and proved to be active in the hydroamination of alkynes.In conclusion, we have extended a knitting polymerization strategy to the field of transition metal complexes, affording a straightforward route to obtain metal-containing polymers and opening up the possibility of heterogenizing many other soluble catalysts.
Experimental
Materials
RuHClCO(PPh3)3 and AuX(PPh3) (X = Cl, NTf2) complexes were prepared according to the literature.31 Triphenylphosphine, biphenyl, iron trichloride, aluminium trichloride, ruthenium trichloride, chloro(tetrahydrothiophene)gold(I) and potassium tetrachloroaurate(III) were obtained from commercial sources.Preparation of polymers
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) with RuCl3·nH2O (160 mg, 0.77 mmol)32 for 48 h. The resulting polymer was washed twice with each solvent in the following order: methanol, water, acetone and tetrahydrofuran. Elemental analysis found: % C 85.4, % H 5.66. TXRF analysis: % Ru 0.02, % P 1.5.
4) with RuCl3·nH2O (160 mg, 0.77 mmol)32 for 48 h. The resulting polymer was washed twice with each solvent in the following order: methanol, water, acetone and tetrahydrofuran. Elemental analysis found: % C 85.4, % H 5.66. TXRF analysis: % Ru 0.02, % P 1.5.
Catalytic activity
Some representative examples of the chromatograms obtained are recorded in Fig. S21–S25.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge MINEICO of Spain MAT2017-82288-C2-2-P project for financial support. AVG thanks Ministerio de Educación y Formación Profesional for FPU17/03463.Notes and references
-
(a) Z. Wang, G. Chen and K. Ding, Self-Supported Catalysts, Chem. Rev., 2009, 109, 322–359 CrossRef CAS PubMed
; (b) Z. Wang, G. Chen and K. Ding, Chem. Rev., 2008, 109, 322–359 CrossRef PubMed
; (c) P. Barbaro and F. Liguori, Heterogenized homogeneous catalysts for fine chemicals production: materials and processes, Springer Science & Business Media, 2010, vol. 33 CrossRef
.
-
(a) H. Zhou, Y. M. Wang, W. Z. Zhang, J. P. Qu and X. B. Lu, Green Chem., 2011, 13, 644–650 RSC
; (b) H. Q. Yang, G. Li, Z. C. Ma, J. B. Chao and Z. Q. Guo, J. Catal., 2010, 276, 123–133 CrossRef CAS
.
-
(a) H. Yang, Y. Wang, Y. Qin, Y. Chong, Q. Yang, G. Li, L. Zhang and W. Li, Green Chem., 2011, 13, 1352 RSC
; (b) K. V. S. Ranganath, J. Kloesges, A. H. Schäfer and F. Glorius, Angew. Chem., Int. Ed., 2010, 49, 7786–7789 CrossRef CAS PubMed
.
- M. Gruttadauria, F. Giacalone and R. Noto, Chem. Soc. Rev., 2008, 37, 1666–1688 RSC
.
-
(a) M. Pintado-Sierra, A. M. Rasero-Almansa, A. Corma, M. Iglesias and F. Sánchez, J. Catal., 2013, 299, 137–145 CrossRef CAS
; (b) A. M. Rasero-Almansa, A. Corma, M. Iglesias and F. Sánchez, Green Chem., 2014, 16, 3522–3527 RSC
; (c) F. J. Song, C. Wang, J. M. Falkowski, L. Q. Ma and W. B. Lin, J. Am. Chem. Soc., 2010, 132, 15390–15398 CrossRef CAS PubMed
.
-
(a) P. Kaur, J. T. Hupp and S. T. Nguyen, ACS Catal., 2011, 1, 819–835 CrossRef CAS
; (b) A. Trewin and A. I. Cooper, Angew. Chem., Int. Ed., 2010, 49, 1533–1535 CrossRef CAS PubMed
; (c) W. Wang, A. Zheng, P. Zhao, Ch. Xia and F. Li, ACS Catal., 2014, 4, 321–327 CrossRef CAS
; (d) D. Wu, F. Xu, B. Sun, R. Fu, H. He and K. Matyjaszewski, Chem. Rev., 2012, 112, 3959–4015 CrossRef CAS PubMed
; (e) Y. Zhang and S. N. Riduan, Chem. Soc. Rev., 2012, 41, 2083–2094 RSC
; (f) L. Tan and B. Tan, Chem. Soc. Rev., 2017, 46, 3322–3356 RSC
.
- S. Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC
.
-
(a) R. K. Totten, M. H. Weston, J. K. Park, O. K. Farha, J. T. Hupp and S. T. Nguyen, ACS Catal., 2013, 3, 1454–1459 CrossRef CAS
; (b) A. M. Shultz, O. K. Farha, J. T. Hupp and S. T. Nguyen, Chem. Sci., 2011, 2, 686–689 RSC
; (c) K. K. Tanabe, N. A. Siladke, E. M. Broderick, T. Kobayashi, J. F. Goldston, M. H. Weston, O. K. Farha, J. T. Hupp, M. Pruski, E. A. Mader, M. J. A. Johnson and S. T. Nguyen, Chem. Sci., 2013, 4, 2483–2489 RSC
.
-
(a) B. Li, R. Gong, W. Wang, X. Huang, W. Zhang, H. Li, C. Hu and B. Tan, Macromolecules, 2011, 44, 2410–2414 CrossRef CAS
; (b) R. T. Woodward, L. A. Stevens, R. Dawson, M. Vijayaraghavan, T. Hasell, I. P. Silverwood, A. V. Ewing, T. Ratvijitvech, J. D. Exley, S. Y. Chong, F. Blanc, D. J. Adams, S. G. Kazarian, C. E. Snape, T. C. Drage and A. I. Cooper, J. Am. Chem. Soc., 2014, 136, 9028–9035 CrossRef CAS PubMed
; (c) L. Li, K. Cai, P. Wang, H. Ren and G. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 201–208 CrossRef CAS PubMed
; (d) L. Li, H. Ren, Y. Yuan, G. Yu and G. Zhu, J. Mater. Chem. A, 2014, 2, 11091–11098 RSC
.
-
(a) K. J. Msayib and N. B. McKeown, J. Mater. Chem. A, 2016, 4, 10110–10113 RSC
; (b) S. Wang, Ch. Zhang, Y. Shu, S. Jiang, Q. Xia, L. Chen, S. Jin, I. Hussain, A. I. Cooper and B. Tan, Sci. Adv., 2017, 3, e1602610 CrossRef PubMed
; (c) E. M. Maya, E. Rangel-Rangel, U. Díaz and M. Iglesias, J. CO2 Util., 2018, 25, 170–179 CrossRef CAS
; (d) J. Guadalupe, A. M. Ray, E. M. Maya, B. Gómez-Lor and M. Iglesias, Polym. Chem., 2018, 9, 4585–4595 RSC
.
- X. Wang, S. Min, S. K. Das, W. Fan, K. Huang and Z. Lai, J. Catal., 2017, 355, 101–109 CrossRef CAS
.
- Ch. Tang, Z. Zou, Y. Fu and K. Song, ChemistrySelect, 2018, 3, 5987–5992 CrossRef CAS
.
- X. Wang, E. A. P. Ling, Ch. Guan, Q. Zhang, W. Wu, P. Liu, N. Zheng, D. Zhang, S. Lopatin, Z. Lai and K.-W. Huang, ChemSusChem, 2018, 11, 3591–3598 CrossRef CAS PubMed
.
- TXRF technique did not allow the quantification of gold since K phosphorous lines and M gold lines are overlapped.
-
(a) H. Li, F. Zhang, Y. Wan and Y. Lu, J. Phys. Chem. B, 2006, 110, 22942 CrossRef CAS PubMed
; (b) W. He, F. Zhang and H. Li, Chem. Sci., 2011, 2, 961–966 RSC
.
-
(a) B. Li, Z. Guan, W. Wang, X. Yang, J. Hu, B. Tan and T. Li, Adv. Mater., 2012, 24, 3390–3395 CrossRef CAS PubMed
; (b) C. Wang, Y. Han, Y. Li, K. Nie, X. Cheng and J. Zhang, RSC Adv., 2016, 6, 34866–34871 RSC
.
-
(a)
D. S. Glueck, Organophosphorus Chemistry: From Molecules to Applications, ed. V. Laroshenko, Wiley-VCH, 2019, p. 457 Search PubMed
; (b) A. C. Vetter, K. Nikitin and D. G. Gilheany Phosphorus, Sulfur, and Silicon and the Related Elements, 2019, DOI:10.1080/10426507.2018.1541242
.
- D. J. Morgan, Surf. Interface Anal., 2015, 47, 1072–1079 CrossRef CAS
.
-
(a) Triphenylphosphine stability: K. Moedritzer and R. E. Miller, J. Therm. Anal., 1969, 1, 151–157 CrossRef CAS
. For gold oxides stability: (b) O. Díaz-Morales, F. Calle-Vallejo, C. de Munck and M. T. M. Koper, Chem. Sci., 2013, 4, 2334–2343 RSC
; (c) A. Kawamoto, H. Ando, H. Ohashi, Y. Kobayashi, T. Honma, T. Ishida, M. Tokunaga, Y. Okaue, S. Utsunomiya and T. Yokoyama, Bull. Chem. Soc. Jpn., 2016, 11, 1385–1390 CrossRef
. For ruthenium oxides stability: (d) J. S. Punni and P. K. Mason, AEAT-1277 Report, Harwell, 1998 Search PubMed.
-
(a) Z. Jia, K. Wang, B. Tan and Y. Gu, Adv. Synth. Catal., 2017, 359, 78–88 CrossRef CAS
; (b) Q. Wang, L. Zhang, L. Hao, Ch. Wang, Q. Wu and Z. Wang, J. Chromatogr. A, 2018, 1575, 18–25 CrossRef CAS PubMed
.
-
(a) A. J. A. Watson and J. M. J. Williams, Science, 2010, 329, 635–636 CrossRef CAS PubMed
; (b) C. Gunanathan and D. Milstein, Science, 2013, 341, 249–260 CrossRef CAS PubMed
.
-
(a) J. P. Adams, Perkin 1, 2000, 1, 125–139 RSC
; (b) J. Gawronski, N. Wascinska and J. Gajewy, Chem. Rev., 2008, 108, 5227–5252 CrossRef CAS PubMed
.
- B. Chen, J. Li, W. Dai, L. Wanga and S. Gao, Green Chem., 2014, 16, 3328–3334 RSC
.
-
(a) A. Corma, J. Navas and M. J. Sabater, Chem. Rev., 2018, 118, 1410–1459 CrossRef CAS PubMed
; (b) K. Sordakis, C. Tang, L. K. Vogt, H. Junge, P. J. Dyson, M. Beller and G. Laurenczy, Chem. Rev., 2018, 118, 372–433 CrossRef CAS PubMed
.
-
(a) B. hen, L. Wang and S. Gao, ACS Catal., 2015, 5, 5851–5876 CrossRef
; (b) M. Mon, R. Adam, J. Ferrando-Soria, A. Corma, D. Armentano, E. Pardo and A. Leyva-Pérez, ACS Catal., 2018, 8, 10401–10406 CrossRef CAS
.
-
W. Kuriyama, Encyclopedia of Reagents for Organic Synthesis, 2014, vol. 1, DOI:10.1002/047084289X.rn01699
.
-
(a) A. Corma, J. Navas and M. J. Sabater, Chem. Rev., 2018, 118, 1410–1459 CrossRef CAS PubMed
; (b) H. Wu, J. Wu and Z. Du, Chin. J. Org. Chem., 2017, 37, 1127–1138 CrossRef CAS
; (c) B. Gnanaprakasam, E. Balaraman, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2011, 50, 12240–12244 CrossRef CAS PubMed
; (d) P. Hu, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2016, 55, 1061–1064 CrossRef CAS PubMed
.
- D. Wang, R. Cai, S. Sharma, J. Jirak, S. K. Thummanapelli, N. G. Akhmedov, H. Zhang, X. Liu, J. L. Petersen and X. Shi, J. Am. Chem. Soc., 2012, 134, 9012–9019 CrossRef CAS PubMed
.
- A. Leyva and A. Corma, J. Org. Chem., 2009, 5, 2067–2074 CrossRef PubMed
.
- L. Huang, M. Arndt, K. Gooßen, H. Heydt and L. J. Gooßen, Chem. Rev., 2015, 115, 2596–2697 CrossRef CAS PubMed
.
-
(a) Synthesis of Ru-complex: A. López, A. Romero, A. Santos, A. Vegas, A. M. Echavarren and P. Noheda, J. Organomet. Chem., 1989, 373, 249–258 CrossRef
. Synthesis of (Ph3P)AuCl; (b) M. I. Bruce, B. K. Nicholson and O. Bin Shawkataly, Inorg. Synth., 1989, 26, 324–328 CAS
. Synthesis of (Ph3P)Au(NTf2); (c) N. Mezailles, L. Ricard and F. Gagosz, Org. Lett., 2005, 7, 4133–4136 CrossRef CAS PubMed
.
- The metal amount was based on the phosphorous loading of the polymer being 0.5% in polymer weight, 1.6 × 10−4 mmol g−1 of polymer.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cy00776h |
| ‡ Present address: Ecole Nationale Supérieure de Chimie de Mulhouse, 3 rue Alfred Werner 68093, Mullhouse, Cedex, France. |
| This journal is © The Royal Society of Chemistry 2019 |