Zinc 2-N-methyl N-confused porphyrin: an efficient catalyst for the conversion of CO2 into cyclic carbonates†
Abstract
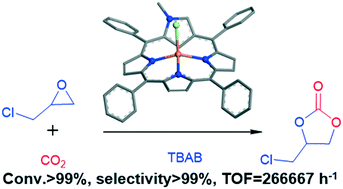
A zinc 2-N-methyl N-confused porphyrin (Zn(NCP)Cl) catalyst was developed for the solvent-free synthesis of cyclic carbonates from epoxides and CO2. Zn(NCP)Cl exhibited very high catalytic activity. Using this catalyst, a series of epoxides have been converted into the corresponding cyclic carbonates in high yields and selectivity (>99%). The turnover frequency (TOF) value reached 266 667 h−1 for the conversion of CO2 with epichlorohydrin into cyclic carbonate at 120 °C and an initial CO2 pressure of 1.7 MPa within 3 h. Furthermore, an almost quantitative cyclic carbonate product was achieved under atmospheric CO2 within 24 h. X-ray structural analysis of Zn(NCP)Cl reveals that the Zn2+ ion is four-coordinate, surrounded by three nitrogen atoms from the N-confused porphyrin and a chlorine atom. Due to the strong electron-withdrawing ability of chloride, zinc shows strong acidity, thereby enhancing its ability to activate epoxide. Density functional theory calculations (DFT) suggest that the ring-opening of epoxide is the rate-determining step of the catalytic cycle.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...