 Open Access Article
Open Access ArticleCobalt pincer complexes for catalytic reduction of nitriles to primary amines†
Jacob
Schneekönig
 ,
Bianca
Tannert
,
Helen
Hornke
,
Matthias
Beller
,
Bianca
Tannert
,
Helen
Hornke
,
Matthias
Beller
 * and
Kathrin
Junge
* and
Kathrin
Junge
 *
*
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany. E-mail: matthias.beller@catalysis.de; kathrin.junge@catalysis.de; Web: http://www.catalysis.de Fax: (+49) 381 1281 5000
First published on 1st April 2019
Abstract
Various cobalt pincer type complexes 1–6 were applied for the catalytic hydrogenation of nitriles to amines. Among these, catalyst 4 is the most efficient, allowing the reduction of aromatic as well as aliphatic nitriles in moderate to excellent yields.
Hydrogenation of nitriles is applied as a valuable method for the preparation of amines, which represent important target molecules in organic chemistry as well as building blocks in life science industries for pharmaceuticals and agrochemicals.1 On the laboratory scale, this transformation is often realized with (over) stoichiometric amounts of sensitive organometallic reagents (e.g. LiAlH4 or NaBH4) and suffers from poor selectivity and waste generation.2 In industry, heterogeneous systems such as RANEY® nickel and RANEY® cobalt are commonly used for the reduction of nitriles but they come along with limited product selectivity or low functional group tolerance.3 Therefore, homogeneous catalytic hydrogenation of nitriles offers a convenient and atom-economic alternative for amine synthesis, operating with higher selectivity and under milder reaction parameters. Compared to the related reduction of imines or nitro compounds,4 this reaction is still less explored. So far, mainly traditional catalyst metals such as Rh,5 Ru,6 Ir,7 Re,8 or Mo9 have been investigated. Due to economic and ecological demands, the replacement of these precious metals by cheaper, more abundant and less toxic 3d metal systems is one of the hot topics in catalysis.10 In accordance with this actual trend, iron11 and manganese12 derived catalysts have been developed for the catalytic hydrogenation of nitriles. In addition to this, cobalt complexes offer an inexpensive alternative to precious metal catalysts due to their low cost and availability. Until recently, compared to iron and manganese involving systems, they were much less investigated.13 Thereby, cobalt compounds, and here especially cobalt pincer type complexes14 show remarkable efficiency in catalytic transformations including polymerization, (transfer) hydrogenation, hydrosilylation/hydroboration as well as N2 or CH activation.13 Although cobalt catalyzed reductions of carbonyl compounds including ketones, aldehydes, or esters15 and of olefins were intensively studied,16 only a few homogeneous cobalt catalysts are known for the selective reduction of nitriles to primary amines (Fig. 1).17 In 2015, the group of Milstein published the first homogeneous Co system for the hydrogenation of nitriles to amines applying the lutidine-based Co pincer complex A (Fig. 1).17a This catalyst bearing a PNNH pincer ligand with a secondary amine side arm allowed potential metal–ligand cooperation (MLC). Although A showed an increased catalytic activity compared to its Co PNP and PNN pincer counterparts,15a the reduction of aromatic nitriles required a relatively high temperature (135 °C) and long reaction time (36–60 hours). Fout and co-workers performed the reduction of aromatic and aliphatic nitriles by applying B under milder reaction conditions (115 °C; 8 h) but without KOtBu, and a secondary imine was formed.17b
In addition, a non-pincer based in situ combination of Co(acac)3 and the tetradentate P-donor ligand (tris[2-(dicyclohexylphosphino)ethyl]phosphine) C was developed by our group.17c Very recently, the Co PN3P pincer complex D17d and the in situ system F17e prepared from CoBr2 and the pincer ligand NH(CH2CH2PiPr2)2 were presented for the cobalt catalyzed hydrogenation of nitriles to the corresponding secondary imines, which normally are only observed as an intermediate within the synthesis of primary amines. Thus, especially regarding the catalyst efficiency and applied reaction parameters, there is still room for improvement.
Based on our previous work on non-noble metal catalysis, especially the application of pincer type complexes,11a,b,12,15d we became interested in the development of a defined Co catalyst with aliphatic pincer ligands for the selective hydrogenation of nitriles. At the start of this project, we tested a selection of cobalt pincer complexes bearing different substituents on the phosphorous atom (1–4) as well as two Co complexes with an N3 donor pincer ligand (5 and 6) (Table 1). The reaction parameters were studied, choosing 4-phenylbenzonitrile (7a) as the model compound for the cobalt-catalysed hydrogenation of nitriles. Full conversion to 4-phenylbenzylamine (8a) was achieved with 5 mol% of the phenyl substituted Co PNP pincer complex 4 in the presence of 20 mol% NaOMe in dioxane at 140 °C and 50 bar H2 after 24 h. After full conversion, formation of black particles was observed. Interestingly, up to 40% conversion to the primary amine 8a and the corresponding intermediate 9a are found by applying the filtered black precipitate in the catalytic test reaction (entry 4). Obviously, the Co PNP pincer complex 4 tends to decompose, forming cobalt nanoparticles which are known to catalyse the nitrile reduction. When catalyst 4 was tested without a base, only small amounts of products 8a and 9a were detected, while NaOMe alone did not show any conversion (entries 2 and 3).
| Entry | Cat. [mol%] | NaOMe [mol%] | T [°C]/t [h] | Conv. [%] | Yield 8ab [%] |
|---|---|---|---|---|---|
| a 0.5 mmol nitrile, 2–5 mol% 1–6, 8–20 mol% NaOMe, 2 mL dioxane, 50 bar H2. b Yield determined with an internal standard. Yield of side product 9a in parentheses. c 8 mol% NaHBEt3. | |||||
| 1 | 4 [5] | (20) | 140/24 | >99 | 96 (3) |
| 2 | 4 [5] | — | 140/24 | 26 | 5 (15) |
| 3 | — | (20) | 140/24 | — | — |
| 4 | Iso. np | — | 140/24 | 40 | 23 (17) |
| 5 | Iso. np | — | 80/6 | — | — |
| 6 | 4 [5] | (20) | 120/18 | >99 | >99 |
| 7 | 4 [5] | (20) | 120/6 | >99 | >99 |
| 8 | 4 [5] | (20) | 100/4 | >99 | >99 |
| 9 | 4 [5] | (20) | 80/4 | >99 | >99 |
| 10 | 4 [4] | (16) | 80/4 | >99 | >99 |
| 11 | 4 [2] | (8) | 80/4 | 52 | 3 (44) |
| 12c | 4 [2] | (8) | 80/4 | >99 | 98 (1) |
| 13c | 1 [2] | (8) | 80/4 | 27 | — |
| 14c | 2 [2] | (8) | 80/4 | 25 | — |
| 15c | 3 [2] | (8) | 80/4 | 21 | — |
| 16c | 5 [2] | (8) | 80/4 | 59 | 50 (6) |
| 17c | 6 [2] | (8) | 80/4 | 60 | 50 (6) |
In order to prevent the decomposition of the homogeneous cobalt catalyst, the benchmark reaction was performed at lower temperature and shorter time (entries 6–10). At 120 °C, a clear red solution was obtained after a 6 h reaction time and the nitrile was completely transformed into a primary amine. Notably, the time and temperature could be further reduced (80 °C, 4 h) and a quantitative yield of 8a was obtained with 4 mol% catalyst loading.
The type of base plays a crucial role in the efficiency of the catalyst. With 2 mol% of Co PNP complex 4 and 8 mol% of NaOMe, the catalytic activity dropped down, while in the presence of 8 mol% NaBHEt3, 98% of 4-phenylbenzylamine (8a) was produced (entries 11 and 12). Finally, Co pincer based complexes bearing different substituents are used under these optimized reaction parameters (2 mol% cat., 8 mol% NaBHEt3, 50 bar H2, 80 °C, 4 h, dioxane). Here, Co PNP complexes (1–3) with i-propyl and cyclohexyl moieties on the phosphorous atom showed little conversion but no product yield (entries 13–15). Also, two Co complexes with an N3 pincer ligand (5 and 6) demonstrated moderate catalytic activity producing 4-phenylbenzylamine (8a) in 50% yield (entries 16 and 17).
Before investigating the scope and limitations of the catalytic system, we studied the real nature of the catalytically active species. As demonstrated in the initial experiments, the homogeneous cobalt complex 4 easily forms Co nanoparticles, which are active, similar to their homogeneous counterparts but with lower selectivity (entry 4). These findings are in agreement with the research works of the groups of Jones15c and Guan,17e who also observed the formation of Co nanoparticles at higher temperatures. Therefore, the kinetic profile of the model reaction was recorded, applying Co PNP pincer complex 4 at 80 °C (Fig. 2). In the case of nanoparticle catalysis, a sigmoidal reaction curve is expected with a significant initiation phase caused by the transformation processes of the catalyst.18 After a heating up period of 10 minutes in the autoclave, the catalytic reaction started forming first the secondary imine 9a which was immediately transformed into the primary amine 8a. The monitoring of the concentration/time relationship for the reduction of 4-phenylbenzonitrile (7a) showed no significant induction period or break in the reaction curve, which is strong evidence for a homogeneously mediated reaction. Nevertheless, two reaction pathways based on a homogeneous catalyst as well as on nanoparticles could proceed in parallel.
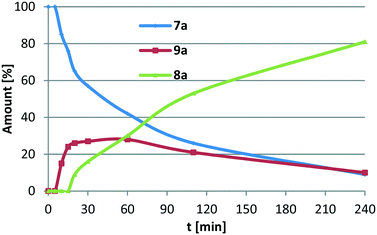 | ||
| Fig. 2 Concentration/time diagram for the hydrogenation of 4-phenylbenzonitrile (7a) catalysed by Co pincer complex 4 at 80 °C. | ||
Additionally, poisoning experiments with suitable scavengers such as PPh3 were performed.19 In the case of a Co nanoparticle catalyzed reaction, sub-stoichiometric amounts of phosphine are expected to block the active sites of the catalyst and suppress the reaction.18 However, the hydrogenation of 4-phenylbenzonitrile in the presence of 1 or 2.5 mol% PPh3 and 5 mol% Co PNP pincer complex 4 at 80 °C formed the primary amine 8a in 97–99% yield (Scheme 1A). In contrast, the reaction of 7a with isolated Co nanoparticles formed in our initial experiments was completely stopped in the presence of 1 mol% PPh3 at 140 °C. These results strongly indicate the homogenous character of the pre-catalyst 4.
Next, catalytic experiments were realized to learn more about the possible mechanism (Scheme 1B). Here, a characteristic aspect of pincer complex based catalysis is the ability of the ligand to play an active role in the reaction.20 This metal–ligand cooperation (MLC) was already observed for iron11a,b or manganese12 catalyzed nitrile reduction including an aliphatic pincer ligand, where the NH moiety was involved in the hydrogen transfer step during the catalysis. When no reaction takes place with the respective pincer catalyst, which is blocked in the NH position, an outer-sphere mechanism is proposed.
Therefore the N-methylated Co pincer complex 10 was applied for the catalytic reduction of 4-phenylbenzonitrile, showing no activity at all.21 Based on this result, we postulated an outer-sphere mechanism for the catalytic hydrogenation of nitriles catalyzed by Co PNP pincer complex 4 (see the ESI†).
Finally, we investigated the general applicability of Co PNP complex 4 (4 mol% catalyst, 16 mol% NaBHEt3, 80 °C, 50 bar H2, 6 h). As shown in Scheme 2, various aromatic and heteroaromatic nitriles were smoothly reduced to the corresponding primary amines in good to excellent yields. Compared to the previously reported Co catalyst systems A–C, these are very mild reaction conditions for nitrile reduction. In particular, para-substituted substrates (7c–h) are reduced in high yields to primary amines; although besides 8d traces of benzylamine were also detected. Nitriles bearing an ortho-substituent on the phenyl ring (7n, 7o, and 7p) gave lower yields compared to the para- or meta-substituted substrates. However, with a longer reaction time (16 hours), the product yields increased to 91–93%. The dinitrile 7t was reduced to the corresponding diamine in 88% yield and 3-cyanopyridine 7l was converted into 70% of amine 8l. Carbonyl moieties are not stable under the applied reaction conditions. Here, hydrogenation of the aldehyde (8i), ketone (8j) and ester groups (8k) took place parallel to the reduction of the nitrile group. Exemplarily, two aliphatic nitriles 7u and 7v were hydrogenated, too. In these cases, a somewhat longer reaction time (16 h) and higher reaction temperature (100 °C) were required to obtain moderately higher product yields.
Conclusions
We have developed a Co catalyst with an aliphatic PNP pincer ligand for the reduction of aromatic and aliphatic nitriles to primary amines. Compared to known cobalt hydrogenation catalysts, complex 4 operates under milder reaction conditions. Mechanistic experiments indicate a homogeneous character of this catalyst system.Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) K. Eller, E. Henkes, R. Rossbacher and H. Höke, in Amines, Aliphatic in Ullman's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000 Search PubMed; (b) S. A. Lawrence, in Amines: Synthesis Properties, and Application, Cambridge University Press, Cambridge, 2006 Search PubMed.
- (a) J. Seyden-Penne, in Reductions by the Alumino- and Borohydrides in Organic Synthesis, Wiley, New York, 1997 Search PubMed; (b) P. G. Andersson and I. J. Munslow, in Modern Reduction Methods, Wiley-VCH, Weinheim, 2008 CrossRef.
- (a) S. Nishimura, in Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis, Wiley, New York, 2001 Search PubMed; (b) M. G. Banwell, M. T. Jones, T. A. Reekie, B. D. Schwartz, S. H. Tan and L. V. White, Org. Biomol. Chem., 2014, 12, 7433 RSC.
- For reviews on homogeneous hydrogenation of nitriles see: (a) S. Gomez, J. A. Peters and T. Maschmeyer, Adv. Synth. Catal., 2002, 344, 1037 CrossRef CAS; (b) S. Das, S. Zhou, D. Addis, S. Enthaler, K. Junge and M. Beller, Top. Catal., 2010, 53, 979 CrossRef CAS; (c) S. Werkmeister, K. Junge and M. Beller, Org. Process Res. Dev., 2014, 18, 289 CrossRef CAS; (d) D. B. Bagal and B. M. Bhanage, Adv. Synth. Catal., 2015, 357, 883 CrossRef CAS; (e) D. S. Merel, M.-L. Tran Do, S. Gaillard, P. Dupau and J.-L. Renaud, Coord. Chem. Rev., 2015, 288, 50 CrossRef CAS; (f) J.-L. Renaud and S. Gaillard, Synthesis, 2016, 48, 3659 CrossRef CAS.
- (a) T. Yoshida, T. Okano and S. Otsuka, J. Chem. Soc., Chem. Commun., 1979, 870 RSC; (b) Y. Sato, Y. Kayaki and T. Ikariya, Organometallics, 2016, 35, 1257 CrossRef CAS.
- (a) R. A. Grey, G. P. Pez, A. Wallo and J. Corsi, J. Chem. Soc., Chem. Commun., 1980, 783 RSC; (b) R. A. Grey, G. P. Pez and A. Wallo, J. Am. Chem. Soc., 1981, 103, 7536 CrossRef CAS; (c) R. P. Beatty and R. A. Paciello, WO Pat., WO/1996/23802-804, 1996 Search PubMed; (d) T. Li, I. Bergner, F. N. Haque, M. Zimmer-De Iuliis, D. Song and R. Morris, Organometallics, 2007, 26, 5940 CrossRef CAS; (e) S. Enthaler, D. Addis, K. Junge, G. Erre and M. Beller, Chem. – Eur. J., 2008, 14, 9491 CrossRef CAS PubMed; (f) S. Enthaler, K. Junge, D. Addis, G. Erre and M. Beller, ChemSusChem, 2008, 1, 1006 CrossRef CAS PubMed; (g) D. Addis, S. Enthaler, K. Junge, B. Wendt and M. Beller, Tetrahedron Lett., 2009, 50, 3654 CrossRef CAS; (h) R. Reguillo, M. Grellier, N. Vautravers, L. Vendier and S. Sabo-Etienne, J. Am. Chem. Soc., 2010, 132, 7854 CrossRef CAS PubMed; (i) C. Gunanathan, M. Hölscher and W. Leitner, Eur. J. Inorg. Chem., 2011, 3381 CrossRef CAS; (j) S. Werkmeister, K. Junge, B. Wendt, A. Spannenberg, H. Jiao, C. Bornschein and M. Beller, Chem. – Eur. J., 2014, 20, 427 CrossRef PubMed; (k) J. Neumann, C. Bornschein, H. Jiao, K. Junge and M. Beller, Eur. J. Org. Chem., 2015, 5944 CrossRef CAS; (l) J.-H. Choi and M. H. G. Prechtl, ChemCatChem, 2015, 7, 1023 CrossRef CAS; (m) R. Adam, C. B. Bheeter, R. Jackstell and M. Beller, ChemCatChem, 2016, 8, 1329 CrossRef CAS; (n) R. Adam, E. Alberico, W. Baumann, H.-J. Drexler, R. Jackstell, H. Junge and M. Beller, Chem. – Eur. J., 2016, 22, 4991 CrossRef CAS PubMed.
- (a) C. Chin and B. Lee, Catal. Lett., 1992, 14, 135 CrossRef CAS; (b) K. Junge, B. Wendt, H. Jiao and M. Beller, ChemCatChem, 2014, 6, 2810 CrossRef CAS.
- K. Rajesh, B. Dudle, O. Blacque and H. Berke, Adv. Synth. Catal., 2011, 353, 1479 CrossRef CAS.
- S. Chakraborty and H. Berke, ACS Catal., 2014, 4, 2191 CrossRef CAS.
- For selected reviews on non-noble metal based catalysis see: (a) K. Junge, K. Schröder and M. Beller, Chem. Commun., 2011, 47, 4849 RSC; (b) P. Dupau, M.-L. Tran Do, S. Gaillard and J.-L. Renaud, Angew. Chem., Int. Ed., 2014, 53, 13004 CrossRef CAS PubMed; (c) D. S. Merel, M.-L. Tran Do, S. Gaillard, P. Dupau and J.-L. Renaud, Coord. Chem. Rev., 2015, 288, 50 CrossRef CAS; (d) L. C. Misal Castro, H. Li, J.-B. Sortais and C. Darcel, Green Chem., 2015, 17, 2283 RSC; (e) T. Zell and D. Milstein, Acc. Chem. Res., 2015, 48, 1979 CrossRef CAS PubMed; (f) M. Garbe, K. Junge and M. Beller, Eur. J. Org. Chem., 2017, 4344 CrossRef CAS.
- (a) C. Bornschein, S. Werkmeister, B. Wendt, H. Jiao, E. Alberico, W. Baumann, H. Junge, K. Junge and M. Beller, Nat. Commun., 2014, 5, 4111 CrossRef CAS PubMed; (b) S. Lange, S. Elangovan, C. Cordes, A. Spannenberg, H. Jiao, H. Junge, S. Bachmann, M. Scalone, C. Topf, K. Junge and M. Beller, Catal. Sci. Technol., 2016, 6, 4768 RSC; (c) S. Chakraborty and D. Milstein, Chem. Commun., 2016, 52, 1812 RSC; (d) S. Chakraborty and D. Milstein, ACS Catal., 2017, 7, 3968 CrossRef CAS.
- S. Elangovan, C. Topf, S. Fischer, H. Jiao, A. Spannenberg, W. Baumann, R. Ludwig, K. Junge and M. Beller, J. Am. Chem. Soc., 2016, 138, 8809 CrossRef CAS PubMed.
- For recent reviews on cobalt catalyzed reduction see: (a) G. A. Filonenko, R. v. Putten, E. J. M. Hensen and E. A. Pidko, Chem. Soc. Rev., 2018, 47, 1459 RSC; (b) W. Liu, B. Sahoo, K. Junge and M. Beller, Acc. Chem. Res., 2018, 51, 1858 CrossRef CAS PubMed; (c) A. Mukherjee and D. Milstein, ACS Catal., 2018, 8, 11435 CrossRef CAS; (d) K. Junge, V. Papa and M. Beller, Chem. – Eur. J., 2019, 25, 122 CrossRef CAS PubMed.
- (a) P. Chirik, in Pincer and Pincer-Type Complexes, ed. K. L. Szabó and O. F. Wendt, Wiley-VCH, Weinheim, 2014, p. 189 Search PubMed; (b) S. Murugesan and K. Kirchner, Dalton Trans., 2016, 45, 416 RSC; (c) F. Kallmeier and R. Kempe, Angew. Chem., Int. Ed., 2018, 57, 46 CrossRef CAS PubMed.
- (a) D. Srimani, A. Mukherjee, A. F. G. Goldberg, G. Leitus, Y. Diskin-Posner, L. J. W. Shimon, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2015, 54, 12357 CrossRef CAS PubMed; (b) T. J. Korstanje, J. I. van der Vlugt, C. J. Elsevier and B. de Bruin, Sciences, 2015, 350, 298 CrossRef CAS PubMed; (c) J. Yuwen, S. Chakraborty, W. W. Brennessel and W. D. Jones, ACS Catal., 2017, 7, 3735 CrossRef; (d) K. Junge, B. Wendt, A. Cingolani, A. Spannenberg, Z. Wei, H. Jiao and M. Beller, Chem. – Eur. J., 2018, 24, 1046 CrossRef CAS PubMed.
- For selected examples of Co-non-pincer based catalysts for the reduction see: (a) C. Federsel, C. Ziebart, R. Jackstell, W. Baumann and M. Beller, Chem. – Eur. J., 2012, 18, 72 CrossRef CAS PubMed; (b) D. Gärtner, A. Welther, B. R. Rad, R. Wolf and A. J. v. Wangelin, Angew. Chem., Int. Ed., 2014, 53, 3722 CrossRef; (c) R. Adam, J. R. Cabrero-Antonino, A. Spannenberg, K. Junge, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2017, 56, 3216 CrossRef CAS PubMed; (d) R. Adam, J. Cabrero-Antonino, K. Junge, R. Jackstell and M. Beller, Catal. Sci. Technol., 2017, 7, 1981 RSC; (e) T. M. Maier, S. Sandl, I. G. Shenderovich, A. Jacobi von Wangelin and R. Wolf, Chem. – Eur. J., 2019, 25, 238 CrossRef CAS PubMed.
- (a) A. Mukherjee, D. Srimani, S. Chakraborty, Y. Ben-David and D. Milstein, J. Am. Chem. Soc., 2015, 137, 8888 CrossRef CAS PubMed; (b) K. Tokmic, B. J. Jackson, A. Salazar, T. J. Woods and A. R. Fout, J. Am. Chem. Soc., 2017, 139, 13554 CrossRef CAS PubMed; (c) R. Adam, C. B. Bheeter, J. R. Cabrero-Antonino, K. Junge, R. Jackstell and M. Beller, ChemSusChem, 2017, 10, 842 CrossRef CAS PubMed; (d) H. Li, A. Al-Dakhil, D. Lupp, S. S. Gholap, Z. Lai, L.-C. Liang and K.-W. Huang, Org. Lett., 2018, 20, 6430 CrossRef CAS PubMed; (e) H. Dai and H. Guan, ACS Catal., 2018, 8, 9125 CrossRef CAS.
- (a) J. A. Widegreen and R. G. Finke, J. Mol. Catal. A: Chem., 2003, 198, 317 CrossRef; (b) R. H. Crabtree, Chem. Rev., 2012, 112, 1536 CrossRef CAS PubMed; (c) J. F. Sonnenberg and R. H. Morris, Catal. Sci. Technol., 2014, 4, 3426 RSC.
- The electrochemical formation of a Co amalgam has been reported only under very selected conditions. Therefore, the addition of Hg(0) to cobalt catalyzed reactions is not an appropriate method to test the occurrence of Co nanoparticles: (a) F. Lihl, Z. Metallkd., 1953, 44, 160 CAS; (b) P. Paklepa, J. Woroniecki and P. K. Wrona, J. Electroanal. Chem., 2001, 498, 181 CrossRef CAS.
- J. R. Khusnutdinova and D. Milstein, Angew. Chem., Int. Ed., 2015, 54, 12236 CrossRef CAS PubMed.
- M. Wang, X. Yu, Z. Shi, M. Qian, K. Jin, J. Chen and R. He, J. Organomet. Chem., 2002, 645, 127 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cy00225a |
| This journal is © The Royal Society of Chemistry 2019 |




