Improving photocatalytic free radical polymerization with hydrochloric acid†
Abstract
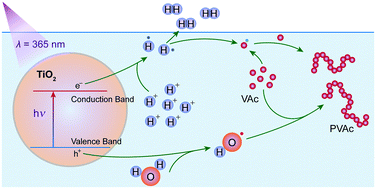
In this work, the photocatalytic free radical polymerization of vinyl acetate (VAc) in aqueous medium was studied by using titanium dioxide nanoparticles as a photoinitiator, and we found the reaction rates and polymer yields of this aqueous photopolymerization to be sensitively influenced by hydrochloric acid (HCl). The chemical structures and molecular weights of the synthesized polyvinyl acetate were characterized by nuclear magnetic resonance (NMR) and gel permeation chromatography. Kinetic studies were carried out to correlate the reaction characteristics with HCl concentration. An efficient photocatalytic free radical polymerization in aqueous medium was thus achieved with the monomer conversion of VAc as high as 89.9%. The electron spin resonance (ESR) results show that OH˙ radicals are generated in the reaction system under UV irradiation, and the intensity of the OH˙ radical signal becomes stronger when HCl is added. Moreover, we used deuterium chloride (DCl) to identify the gaseous products of this photocatalytic reaction. As proved by the isotope analysis and gas chromatograph-mass spectrometry, HD and D2 were generated markedly. The 2H-NMR spectrum revealed that DCl had participated in the photocatalytic initiation reactions. According to these results, the initiation reactions were analyzed in terms of the reduction reactions concerning H+ and D+.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...