Metal substituted pyrochlore phase LixLa2−xCe1.8Ru0.2O7−δ (x = 0.0–0.6) as an effective catalyst for oxidative and auto-thermal steam reforming of ethanol†
Abstract
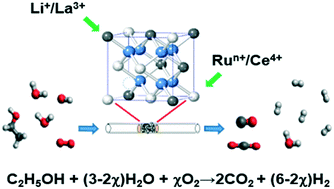
Pyrochlore phase LixLa2−xCe1.8Ru0.2O7−δ (x = 0.0–0.6) [LLCRO] substituted by Li and Ru in A and B sites was synthesized and studied for performance in oxidative steam and auto-thermal reforming of ethanol (OSRE, ATR). The samples were characterized by powder X-ray diffraction (PXRD), X-ray absorption spectroscopy (XAS), and X-ray photoelectron spectroscopy (XPS) to examine the effect of substituted Li+ and Run+ on OSRE performance. The results indicated that the substituted Li ions modified the oxidation states of Ru and Ce in LLCRO, which led to oxygen vacancies/mobility and played an important role in the catalytic performance of OSRE. Using LLCRO as the catalyst and supported by Al2O3, La2Zr2O7 (LZO) was investigated for its hydrogen production performance. The catalytic stability of the LLCRO (x = 0.6)/LZO catalysts was tested at 350 °C under oxidative steam conditions (OSRE) and showed a stable ethanol conversion and hydrogen production (XEtOH = 98(3)%; SH2 = 95(3)%). Under autothermal conditions (ATR), the LLCRO (x = 0.6) catalyst supported by LZO exhibited a stable catalytic performance with an average ethanol conversion and hydrogen selectivity of 90(3)% and 71(4)%, respectively. Carbon deposition was significantly suppressed during the OSRE and ATR processes on LLCRO (x = 0.6) catalysts supported by LZO, whereas, a significant carbon deposition was observed for LLCRO (x = 0.6)/Al2O3 as the catalyst. These results indicate that LLCRO (x = 0.6) supported by LZO is an effective catalyst for hydrogen production. The enhanced activity was attributed to the effectively suppressed carbon deposition and the sintering of highly dispersed Ru ions in the LLCRO catalyst, which may be due to the strong binding between the catalyst and LZO.


 Please wait while we load your content...
Please wait while we load your content...