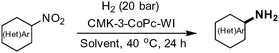
Superior activity and selectivity of heterogenized cobalt catalysts for hydrogenation of nitroarenes†
Wu
Li‡
 a,
Jens
Artz‡
a,
Jens
Artz‡
 b,
Cornelia
Broicher
b,
Kathrin
Junge
a,
Heinrich
Hartmann
c,
Astrid
Besmehn
b,
Cornelia
Broicher
b,
Kathrin
Junge
a,
Heinrich
Hartmann
c,
Astrid
Besmehn
 c,
Regina
Palkovits
c,
Regina
Palkovits
 *b and
Matthias
Beller
*b and
Matthias
Beller
 *a
*a
aLeibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein Straße 29a, Rostock, 18059, Germany. E-mail: Matthias.Beller@catalysis.de
bInstitut für Technische und Makromolekulare Chemie, RWTH Aachen University, Worringerweg 2, 52074 Aachen, Germany. E-mail: Palkovits@itmc.rwth-aachen.de
cForschungszentrum Jülich GmbH, Wilhelm-Johnen-Straße, 5248 Jülich, Germany
First published on 29th November 2018
Abstract
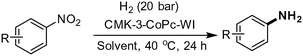
The development of improved catalysts for highly selective hydrogenation of nitroarenes is described. For this purpose Co nanoparticles were supported on ordered mesoporous carbon CMK-3 and characterized in detail. The optimal CMK-3-CoPc catalyst exhibits excellent hydrogenation activity for several (hetero)aromatic nitro compounds and yielded the corresponding anilines under mild conditions (40 °C, 20 bar H2).
Introduction
Catalytic hydrogenation of nitroarenes, either in vapor or liquid phase, is a valuable method to synthesize intermediates for dyes, pigments, optical brighteners, pharmaceuticals, agrochemicals and monomers for polyurethanes.1 In general, these processes are highly exothermic (>550 kJ mol−1 in liquid phase); thus, there is an increasing interest performing such hydrogenations at lower temperatures and pressures even for large-scale production.2 Obviously, for functionalized substrates, the use of milder reaction conditions is also advantageous with respect to selectivity and to avoid undesired side-reactions.3In the past two decades, the use of supported metal nanoparticles (NPs) emerged as an innovative tool in catalysis.4 As an example, precious metal-based nanoparticles, such as Pt, Pd, Rh, Ru and Au, are nowadays routinely used for hydrogenation of nitroarenes.1f,5 Similar to molecularly-defined complexes, the activity and selectivity of such supported nanoparticles can be fine-tuned by means of their chemical surrounding. It was shown that carbonizing organometallic complexes enables strong metal–support interactions, thereby influencing the electron density and steric environment of the active sites.6 Following this concept, earth-abundant metals embedded in nitrogen-doped carbon materials have been employed in selective reductions of nitroarenes, nitriles, aldehydes, and ketones as well as oxidation reactions.7–9 General drawbacks of most of these transformations are the relatively high temperatures (>100 °C) and/or pressures (50 bar H2) required. Hence, hydrogenations of nitroarenes under milder conditions using earth-abundant metal catalysts remain a challenging task in this field. To improve their performance, we had the idea to increase the accessibility of the active sites by further tuning the structural properties of the solid support, e.g. porosity and specific surface area.10 More specifically, using mesoporous materials with ultrahigh surface areas, large pore volumes and tunable pore sizes and shapes should constitute promising supports for improved catalysis.11
Here, we report a facile synthesis of tailor-made porous and carbonaceous Co-phthalocyanine (CoPc) based nanoparticles. As the optimal structure type, ordered mesoporous carbon (CMK-3-type) materials were attempted,12 derived from ordered mesoporous silica (SBA-15) as template.13 The optimal CMK-3-CoPc catalyst showed high activity for the hydrogenation of a broad range of (hetero)aromatic nitroarenes under mild conditions.
Results and discussion
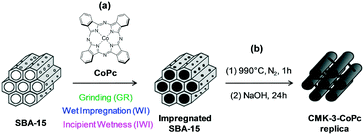
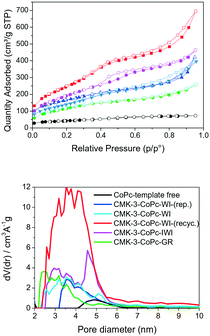
At the start of our investigations, ordered mesoporous carbon materials (CMK-3) were prepared by adopting a procedure de-scribed by Ryoo et al.12 For this purpose, we applied different methods to impregnate mesoporous silica SBA-15 (hard-template) with cobalt phthalocyanine (CoPc) (see ESI2.2.1–2.2.3†). The resulting material was dried at 100 °C and 350 °C for 1 h and subsequently carbonized at 990 °C for 1 h under nitrogen atmosphere. To remove the silica template from the silica Co phthalocyanine composite, the resulting black powder was treated with 5 M NaOH solution at 100 °C for 24 h. Centrifugation of the solution, decantation, and washing with distilled water was repeated ten times to ensure complete elimination of the silica. The obtained ordered mesoporous carbon was finally dried (100 °C) and is denoted as CMK-3-CoPc (Scheme 1). ICP-OES of this material revealed that <5 wt% of Si remained within the porous carbon network. For comparison, template-free CoPc was pyrolyzed under identical conditions in order to investigate structure–activity correlations. The detailed syntheses of the materials are available in the ESI.†Thermogravimetric analysis (TGA) indicates a high thermal stability (>350 °C) of the structured CMK-3-CoPc (Fig. S1†). N2-physisorption measurements (Fig. 1, Table 1) reveal no pronounced porosity and only a marginal specific surface area (SBET) for the template-free carbonized CoPc material. In contrast, all templated CMK-3-CoPc catalysts exhibit increased porosities ranging from 0.49–0.62 cm3 g−1 at surface areas in-between 342 and 522 m2 g−1 (Table 1, entries 3–6). Thus, all preparation techniques for structured CMK-3-CoPc catalysts lead to comparable structural properties, although mesoporosity appears to decline slightly in the order of incipient wetness (IWI) to wet impregnation (WI) and further to physical grinding (GR). This might be assigned to incomplete filling of the pore system of SBA-15, leading to less developed structures during carbonization. ICP-OES analysis confirms cobalt loadings in the range of 5.8 to 7.0 wt.% within all structured CMK-3-CoPc systems. This value is slightly lower compared to the cobalt content of untreated CoPc (8.9 wt%, Table 1, entry 1) and might be attributed to partial loss during SBA-15 template removal (5 M NaOH, 100 °C, 24 h).
| Entry | Catalyst | S BET [m2 g−1] | V Ptotal [cm3 g−1] | D P [nm] | CoICP [wt%] |
|---|---|---|---|---|---|
| 1 | CoPc (as received) | — | — | — | 8.9 |
| 2 | CoPc (template free) | 116 | 0.10 | 5.0 | 7.8 |
| 3 | CMK-3-CoPc-GR | 342 | 0.49 | 3.0 | 6.1 |
| 4 | CMK-3-CoPc-IWI | 522 | 0.62 | 4.4 | 7.0 |
| 5 | CMK-3-CoPc-WI | 466 | 0.61 | 4.4 | 6.1 |
| 6 | CMK-3-CoPc-WI-rep. | 442 | 0.56 | 3.8 | 5.9 |
| 7 | CMK-3-CoPc-WI-recyc. | 931 | 1.07 | 4.0 | 5.8 |
The rather harsh treatment, however, leads to the assumption, that any residual Co-species are well embedded and thus strongly incorporated within the carbon structure, potentially preventing leaching and subsequent catalyst deactivation. A slight loss in Co content is observable for the template-free carbonized CoPc structure (Table 1, entry 2), which might be assigned to volatile Co-species formed under synthesis conditions. Reproducibility for wet impregnation as a facile preparation technique was also studied, and comparable structure as well as cobalt contents were confirmed (Table 1, entry 5 vs. 6).
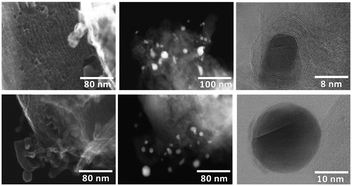
Powder XRD patterns of the template-free and structured materials mainly exhibit the C(002) reflections characteristic for graphitic carbon, while no clear reflections for Co0 nanoparticles are observed (Fig. 2). In contrast, nanoparticle formation is clearly confirmed by STEM imaging of the CMK-3-CoPc-WI sample (Fig. 3). HAADF-STEM measurements reveal both finely distributed Co-species throughout the whole CMK-3 material as well as nanoparticles varying broadly in size from approximately 5 to 20 nm. All cobalt nanoparticles are embedded within a carbon shell, varying slightly in layer thickness. Furthermore, the typical ordered porous CMK-3 structure becomes evident. XPS analysis (see supporting information, curve fitting was performed according to Biesinger et al.14) of template-free CoPc and CMK-3-CoPc-WI reveals Co, O and N species in the presence of a pronounced graphitic sp2-hybridized C 1s signal, which conforms to the results from XRD analysis. The O 1s signal can mostly be assigned to aromatic and aliphatic C–O bonding as well as adsorbed water and O2 on the catalyst surface. All samples consist mostly of graphitic and pyridinic N species, while only small amounts of oxidized N is present. In addition, the unstructured CoPc (template-free) contains more pyridinic than graphitic N and reveals a significant amount of CoO species, which only occur in trace quantities for the structured CMK-3-CoPc-WI catalysts. From the Co 2p3/2 spectra, CoO is confirmed as the major species compared to metallic Co(0) in unstructured CoPc, while structured CMK-3-CoPc-WI furthermore contains large amounts of Co(OH)2. A precise statement regarding the exact nature of the catalytically active site can therefore not be made at this point and will be part of future investigations.
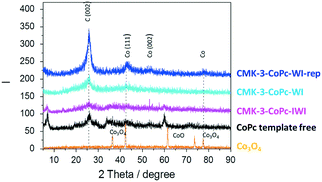 | ||
| Fig. 2 XRD analysis of template-free carbonized CoPc (black), CMK-3-CoPc-IWI (magenta) and CMK-3-CoPc-WI (light blue and reproduced, blue) in comparison to Co3O4. | ||
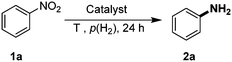
Initial catalytic tests were conducted with nitrobenzene (1a) as industrially relevant substrate in the solvent mixture 2-methyltetrahydrofuran/H2O. In order to identify outperforming catalysts, we conducted experiments at low temperature (40 °C) and pressure (20 bar hydrogen). To our delight, the desired aniline (2a) is obtained in >99% yield at full conversion in the presence of CMK-3-CoPc-WI (Table 2, entry 1). For comparison, the template-free and non-structured catalyst CoPc yielded the product in only 67% (Table 2, entry 2), while simple Co(OAc)2 carbonized under the same conditions but in the absence of phthalocyanine showed much lower activity with only 20% yield of aniline (Table 2, entry 3). Apparently, both structuring and N-doping of the material has a significant effect on its catalytic performance. Thus, using CMK-3-CoPc-GR synthesized via grinding and subsequent carbonization gave somewhat lower yield of aniline in comparison to CMK-3-CoPc-WI (Table 2, entry 4 vs. 1). On the other hand, 97% yield of aniline was achieved using CMK-3-CoPc-IWI prepared via incipient wetness impregnation prior to carbonization (Table 2, entry 5). Comparing all three CMK-3-CoPc catalysts prepared via different synthesis procedures, there seems to be no clear correlation between specific surface area, porosity and catalytic activity. The most pronounced difference in activity is observed for the catalyst CMK-3-CoPc-GR prepared by physical grinding.
| Entry | Catalyst | T (°C) | p (bar) | Conv. (%) | Yielda (%) |
|---|---|---|---|---|---|
| Reaction conditions: 0.5 mmol nitrobenzene, 6.2 mol% [Co] (30 mg), 1.5 mL 2-methyltetrahydrofuran and 0.5 mL H2O.a GC conversions and selectivities using n-dodecane as an internal standard;b 16 h. | |||||
| 1 | CMK-3-CoPc-WI | 40 | 20 | 100 | >99 |
| 2 | CoPc (template-free) | 40 | 20 | 68 | 67 |
| 3 | Co(OAc)2 carbonized (template-free) | 40 | 20 | 20 | 20 |
| 4 | CMK-3-CoPc-Gr | 40 | 20 | 73 | 72 |
| 5 | CMK-3-CoPc-IWI | 40 | 20 | 99 | 97 |
| 6 | CMK-3-CoPc-WI | 40 | 20 | 83 | 81b |
| 7 | CMK-3-CoPc-WI | 40 | 10 | 65 | 63 |
| 8 | Pd/C | 40 | 20 | 81 | 80 |
| 9 | CMK-3-CoPc-WI | RT | 20 | 48 | 48 |
Since all three structured catalysts exhibit comparable specific surface areas and pore volumes, its comparably low activity can be correlated to the small number of mesopores within CMK-3-CoPc-GR. Wet impregnation methods seem to be more suitable to introduce the precursor into the pore system of SBA-15 and replicate it, yielding a partially mesoporous material. The template-free and thus unstructured CoPc showed a similar structure–activity effect. Even though the template-free CoPc already provides a specific surface area of 150 m2 g−1, the obtained material is fully microporous with a total pore volume of only 0.12 cm3 g−1. Thus, we conclude that transport pores in the mesoporous regime are beneficial for the overall catalytic performance due to enhanced accessibility of the active sites. Interestingly, the model reaction proceeded well already at 10 bar H2 pressure (Table 2, entry 7) and even at room temperature; albeit the conversion was lower (Table 2, entry 9). This latter example constitutes one of the rare examples of nitro hydrogenation with an earth-abundant metal catalyst at ambient temperature. Comparing the activity of CMK-3-CoPc-WI with a benchmark Pd/C (10 wt% Pd) catalyst under the standard conditions, our system outperforms the noble metal catalyst (Table 2, entry 1 vs. entry 8). Next, we investigated the substrate scope and long-term stability of the best catalyst system.
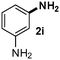
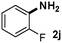
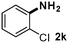
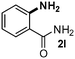
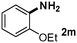
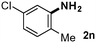
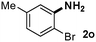
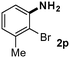
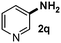
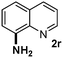
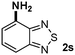
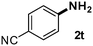
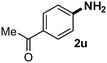
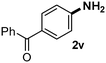
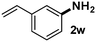
CMK-3-CoPc-WI catalyzes the hydrogenation of a wide range of substrates containing diverse substituent groups under mild conditions (Table 3). For example, nitroarenes containing electron-donating groups, e.g. methyl (1b), methoxy (1e), ethoxy (1f), and amino (1i) gave high yields (>90%) of the desired products. A similar behavior is observed with arenes substituted with electron-withdrawing groups such as carbamoyl (1l) and trifluoromethyl (1g). A variety of industrially relevant anilines such as toluidines (2b), chloro- (2c), and fluoroanilines (2j), were prepared in excellent yields (90–99%), too. Notably, no dehalogenation products were detected for fluoro, chloro and bromo-substituted substrates (2c, 2d, 2k, 2n and 2o). In general, nitrogen- and/or sulfur-containing heteroaromatic amines are valuable building blocks for the preparation of agrochemicals and pharmaceuticals. Therefore, different nitroheteroarenes 1q, 1r and 1s were subjected to the standard hydrogenation conditions, and all three substrates were reduced in excellent yields (98–99%). In order to study the chemoselectivity of the present system in more detail, we explored the hydrogenation of nitroarenes bearing other reducible groups (Table 4). Gratifyingly, the CMK-3-CoPc-WI catalyst hydrogenated the nitro group selectively in the presence of cyano, ketone, and alkenyl groups (2t, 2u and 2v). Most interestingly, the catalyst is able to reduce nitro groups in the presence of alkenes, with full conversion and without any detectable reduction of the unsaturated unit (2w–2y).
Both stability as well as reusability are important parameters for the application of heterogeneous catalysts. Hence, we explore the recyclability of CMK-3-CoPc-WI using nitrobenzene as the substrate. As depicted in Fig. 4, after five successive cycles the desired product aniline was obtained in 89% yield. No significant cobalt leaching could be identified (ICP-OES analysis, see ESI†). In addition, the Co content within the material remains unchanged (Table 1, entry 5 versus 7). Interestingly, N2-physisorption reveals an increase in both specific surface area and total pore volume upon recycling. There is no clear indication, where this restructuring derives from; however, the catalytic performance is only slightly affected. From STEM images of the recycled catalyst, no drastic change in the materials morphology can be confirmed, as the CMK-3 structure is preserved over five successive runs and no obvious change in nanoparticle size is observable, either (Fig. S5†). We assume that the embedment of the active sites within the structured support as well as the inherent formation of a carbon shell provides a stabilizing effect during catalysis and therefore preserves the nanoparticles from further agglomeration, leaching and subsequent deactivation. In addition, XPS analysis after the reaction reveals that Co-species are only slightly oxidized further, mostly to Co(OH)2 species, which is to be expected in the presence of water under reaction conditions. These results demonstrate that our cobalt catalyst is generally stable during the course of nitro hydrogenation. Finally, it should also be mentioned that this stable and practical cobalt material can be conveniently handled under air without pronounced deactivation.
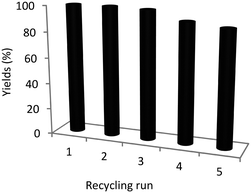 | ||
| Fig. 4 Recycling experiments using CMK-3-CoPc-WI. Yield of aniline was determined by GC using n-dodecane as internal standard. | ||
Conclusion
In summary, we have prepared a highly active Co-catalyst via structuring of cobalt phthalocyanine upon impregnation into SBA-15 and subsequent carbonization at 990 °C. Hard-templating enabled the synthesis of well-structured CMK-3-CoPc catalysts with significantly enhanced activity due to improved accessibility of the active sites. The type of impregnation influences the resulting catalyst performance significantly. In this respect, wet impregnation led to the best results, which is attributed to the pronounced content of mesopores derived from improved SBA-15 replication, thus facilitating accessibility of the active sites within the catalyst. Applying the optimal CMK-3-CoPc-WI catalyst, a wide range of (hetero)aromatic nitro compounds has been hydrogenated to the corresponding anilines under very mild conditions in excellent yields. Notably, the catalyst showed high chemoselectivity and sensitive groups such as halogens and reducible functional moieties, e.g. nitriles, ketones, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds, are well tolerated.
C double bonds, are well tolerated.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank S. Buchholz for her excellent analytical support as well as the State of Mecklenburg-Western Pomerania for financial support. Regina Palkovits and Cornelia Broicher acknowledge the Federal Ministry of Education and Research (BMBF) for funding part of this work with the MANGAN research cluster (FKZ 03SF0508).Notes and references
- (a) H. A. Wittcoff, B. G. Reuben and J. S. Plotkin, in Industrial Organic Chemicals, Wiley-Interscience, New York, 2nd edn, 2004 CrossRef; (b) R. S. Downing, P. J. Kunkeler and H. van Bekkum, Catal. Today, 1997, 37, 121–136 CrossRef CAS; (c) H.-U. Blaser, H. Steiner and M. Studer, ChemCatChem, 2009, 1, 210–221 CrossRef CAS; (d) P. Serna and A. Corma, ACS Catal., 2015, 5, 7114–7121 CrossRef CAS; (e) J. Song, Z.-F. Huang, L. Pan, K. Li, X. Zhang, L. Wang and J.-J. Zou, Appl. Catal., A, 2018, 227, 386–408 CrossRef CAS; (f) H. Goksu, H. Sert, B. Kilbas and F. Sen, Curr. Org. Chem., 2017, 21, 794–820 CrossRef CAS; (g) M. Pietrowski, Curr. Org. Chem., 2012, 9, 470–487 CAS.
- P. F. Vogt and J. J. Gerulis, Amines, Aromatic Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 2002 Search PubMed.
- (a) B. Sahoo, A. E. Surkus, M. M. Pohl, J. Radnik, M. Schneider, S. Bachmann, M. Scalone, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2017, 56, 11242–11247 CrossRef CAS PubMed.
- (a) A. T. Bell, Science, 2003, 299, 1688–1691 CrossRef CAS PubMed; (b) R. Schlogl and S. B. Abd Hamid, Angew. Chem., Int. Ed., 2004, 43, 1628–1637 CrossRef PubMed; (c) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7872 CrossRef CAS PubMed.
- (a) A. Grirrane, A. Corma and H. Garcia, Science, 2008, 322, 1661–1664 CrossRef CAS PubMed; (b) A. Corma, P. Serna, P. Concepcion and J. J. Calvino, J. Am. Chem. Soc., 2008, 130, 8748–8753 CrossRef CAS PubMed; (c) H. Wei, Y. Ren, A. Wang, X. Liu, X. Liu, L. Zhang, S. Miao, L. Li, J. Liu, J. Wang, G. Wang, D. Su and T. Zhang, Chem. Sci., 2017, 8, 5126–5131 RSC; (d) S. Zhang, C. R. Chang, Z. Q. Huang, J. Li, Z. Wu, Y. Ma, Z. Zhang, Y. Wang and Y. Qu, J. Am. Chem. Soc., 2016, 138, 2629–2637 CrossRef CAS PubMed; (e) F. Leng, I. C. Gerber, P. Lecante, S. Moldovan, M. Girleanu, M. R. Axet and P. Serp, ACS Catal., 2016, 6, 6018–6024 CrossRef CAS; (f) M. Boronat, P. Concepcion, A. Corma, S. Gonzalez, F. Illas and P. Serna, J. Am. Chem. Soc., 2007, 129, 16230–16237 CrossRef CAS PubMed; (g) J. Zhang, L. Wang, Y. Shao, Y. Wang, B. C. Gates and F. S. Xiao, Angew. Chem., Int. Ed., 2017, 56, 9747–9751 CrossRef CAS PubMed.
- (a) G. A. Somorjai, H. Frei and J. Y. Park, J. Am. Chem. Soc., 2009, 131, 16589–16605 CrossRef CAS PubMed; (b) G. A. Somorjai and J. Y. Park, Top. Catal., 2008, 49, 126–135 CrossRef CAS; (c) C. K. Tsung, J. N. Kuhn, W. Huang, C. Aliaga, L. I. Hung, G. A. Somorjai and P. Yang, J. Am. Chem. Soc., 2009, 131, 5816–5822 CrossRef CAS PubMed.
- (a) R. V. Jagadeesh, A. E. Surkus, H. Junge, M. M. Pohl, J. Radnik, J. Rabeah, H. Huan, V. Schunemann, A. Bruckner and M. Beller, Science, 2013, 342, 1073–1076 CrossRef CAS PubMed; (b) F. Chen, C. Topf, J. Radnik, C. Kreyenschulte, H. Lund, M. Schneider, A. E. Surkus, L. He, K. Junge and M. Beller, J. Am. Chem. Soc., 2016, 138, 8781–8788 CrossRef CAS PubMed; (c) X. Cui, Y. Li, S. Bachmann, M. Scalone, A. E. Surkus, K. Junge, C. Topf and M. Beller, J. Am. Chem. Soc., 2015, 137, 10652–10658 CrossRef CAS PubMed.
- (a) M. Li, F. Xu, H. Li and Y. Wang, Catal. Sci. Technol., 2016, 6, 3670–3693 RSC; (b) L. He, F. Weniger, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2016, 55, 12582–12594 CrossRef CAS PubMed; (c) Y. Cao, S. Mao, M. Li, Y. Chen and Y. Wang, ACS Catal., 2017, 7, 8090–8112 CrossRef CAS.
- (a) I. Sorribes, L. Liu and A. Corma, ACS Catal., 2017, 7, 2698–2708 CrossRef CAS; (b) Z. Wei, J. Wang, S. Mao, D. Su, H. Jin, Y. Wang, F. Xu, H. Li and Y. Wang, ACS Catal., 2015, 5, 4783–4789 CrossRef CAS; (c) T. Schwob and R. Kempe, Angew. Chem., Int. Ed., 2016, 55, 15175–15179 CrossRef CAS PubMed; (d) L. Liu, P. Concepción and A. Corma, J. Catal., 2016, 340, 1–9 CrossRef CAS; (e) B. Sahoo, D. Formenti, C. Topf, S. Bachmann, M. Scalone, K. Junge and M. Beller, ChemSusChem, 2017, 10, 3035–3039 CrossRef CAS PubMed; (f) L. Liu, F. Gao, P. Concepción and A. Corma, J. Catal., 2017, 350, 218–225 CrossRef CAS; (g) F. Zhang, C. Zhao, S. Chen, H. Li, H. Yang and X.-M. Zhang, J. Catal., 2017, 348, 212–222 CrossRef CAS; (h) P. Zhou, L. Jiang, F. Wang, K. Deng, K. Lv and Z. Zhang, Sci. Adv., 2017, 3, e1601945 CrossRef PubMed.
- (a) J. Panpranot, Catal. Today, 2002, 77, 269–284 CrossRef CAS; (b) A. n. Martıínez, C. López, F. Márquez and I. Díaz, J. Catal., 2003, 220, 486–499 CrossRef; (c) G. Prieto, A. Martínez, P. Concepción and R. Moreno-Tost, J. Catal., 2009, 266, 129–144 CrossRef CAS; (d) K. Na, S. Alayoglu, R. Ye and G. A. Somorjai, J. Am. Chem. Soc., 2014, 136, 17207–17212 CrossRef CAS PubMed; (e) J. Artz, S. Mallmann and R. Palkovits, ChemSusChem, 2015, 8, 672–679 CrossRef CAS PubMed; (f) D. Gu, C. J. Jia, C. Weidenthaler, H. J. Bongard, B. Spliethoff, W. Schmidt and F. Schuth, J. Am. Chem. Soc., 2015, 137, 11407–11418 CrossRef CAS PubMed.
- (a) C.-M. Yang, H.-A. Lin, B. Zibrowius, B. Spliethoff, F. Schüth, S.-C. Liou, M.-W. Chu and C.-H. Chen, Chem. Mater., 2007, 19, 3205–3211 CrossRef CAS; (b) Y. Meng, D. Gu, F. Zhang, Y. Shi, H. Yang, Z. Li, C. Yu, B. Tu and D. Zhao, Angew. Chem., Int. Ed., 2005, 44, 7053–7059 CrossRef CAS PubMed; (c) J. Wei, D. Zhou, Z. Sun, Y. Deng, Y. Xia and D. Zhao, Adv. Funct. Mater., 2013, 23, 2322–2328 CrossRef CAS; (d) A. Eftekhari and Z. Fan, Mater. Chem. Front., 2017, 1, 1001–1027 RSC; (e) D. Gu, W. Schmidt, C. M. Pichler, H. J. Bongard, B. Spliethoff, S. Asahina, Z. Cao, O. Terasaki and F. Schuth, Angew. Chem., Int. Ed., 2017, 56, 11222–11225 CrossRef CAS PubMed; (f) K. T. Lee, X. Ji, M. Rault and L. F. Nazar, Angew. Chem., Int. Ed., 2009, 48, 5661–5665 CrossRef CAS PubMed.
- L. A. Solovyov, A. N. Shmakov, V. I. Zaikovskii, S. H. Joo and R. Ryoo, Carbon, 2002, 40, 2477–2481 CrossRef CAS.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS PubMed.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. St. C. Smart, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cy01634h |
| ‡ These two authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |