Wearable and flexible electronics for continuous molecular monitoring
Abstract
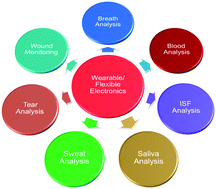
Wearable biosensors have received tremendous attention over the past decade owing to their great potential in predictive analytics and treatment toward personalized medicine. Flexible electronics could serve as an ideal platform for personalized wearable devices because of their unique properties such as light weight, low cost, high flexibility and great conformability. Unlike most reported flexible sensors that mainly track physical activities and vital signs, the new generation of wearable and flexible chemical sensors enables real-time, continuous and fast detection of accessible biomarkers from the human body, and allows for the collection of large-scale information about the individual's dynamic health status at the molecular level. In this article, we review and highlight recent advances in wearable and flexible sensors toward continuous and non-invasive molecular analysis in sweat, tears, saliva, interstitial fluid, blood, wound exudate as well as exhaled breath. The flexible platforms, sensing mechanisms, and device and system configurations employed for continuous monitoring are summarized. We also discuss the key challenges and opportunities of the wearable and flexible chemical sensors that lie ahead.
- This article is part of the themed collection: Materials chemistry in flexible electronics


 Please wait while we load your content...
Please wait while we load your content...