Open Access Article
Open Access ArticleDoping homogeneity in co-doped materials investigated at different length scales†
Wenyu
Li
 a,
Philippe F.
Smet
a,
Philippe F.
Smet
 *b,
Lisa I. D. J.
Martin
*b,
Lisa I. D. J.
Martin
 b,
Christian
Pritzel
c and
Jörn
Schmedt auf der Günne
b,
Christian
Pritzel
c and
Jörn
Schmedt auf der Günne
 *a
*a
aInorganic Materials Chemistry, University of Siegen, Adolf-Reichwein-Str. 2, 57076 Siegen, Germany. E-mail: gunnej@chemie.uni-siegen.de
bDepartment of Solid State Sciences, Ghent University, Krijgslaan 281-S1, 9000 Gent, Belgium. E-mail: Philippe.Smet@UGent.be
cConstruction and Materials Chemistry, University of Siegen, Paul-Bonatz-Str. 9-11, 57076 Siegen, Germany
First published on 4th December 2019
Abstract
Doping homogeneity is important for the properties of co-doped phosphors, as it can affect the energy transfer between sensitizer and activator ions. In a case study we apply different methods, that is scanning electron microscopy (SEM) combined with energy dispersive X-ray spectroscopy (EDX) mapping, SEM combined with cathodoluminescence (CL) and solid-state nuclear magnetic resonance (NMR), to study the doping homogeneity of the host system monazite LaPO4 doped with two different lanthanide ions on different length scales. A new criterion for doping heterogeneity in co-doped systems is developed, which is based on the NMR visibility function, which for this purpose is extended to doping with two or more paramagnetic dopants. A deviation from this function is indicative of doping heterogeneity on the length-scale of the blind-spheres of the paramagnetic dopants. A discussion of the advantages and disadvantages of the different methods is presented. The combined approach allows to study doping homogeneity from the nm to the μm scale.
Introduction
Co-doping is often utilized to achieve extra performance for functional materials. For example, defect populations can be manipulated by co-doping in semi-conductors1 to achieve changes in conductivity or in luminescence materials to achieve higher efficiency,2 brightness or longer afterglow.3 For glass lasers4 co-doping makes a higher dopant-solubility and thermal tolerance possible. Not only the choice of suitable pairs of co-dopants needs to be considered depending on the application scenario, but also the distribution of the co-dopants in the host material is important. Co-doped afterglow materials for example critically depend on energy transfer5,6 processes between different dopant ions which are limited by their distance. As an example, the addition of Dy3+ to the SrAl2O4:Eu2+ persistent phosphor increases the brightness and the duration of the afterglow.7 However, partial segregation of dysprosium has been observed at grain boundaries, affecting the actual dopant concentration inside the phosphor particles.8In general the concept homogeneity depends on a length scale or a volume.9 For example a glass sample may appear homogeneous when investigated by visible light but heterogeneous on an atomic scale. In case of co-doped phosphors the interesting length scale is of the order of the energy transfer distance (up to a few nm, depending on the transfer mechanism).5,6 In the present case we refer to “homogeneous doping” if the La atoms in the host material are statistically replaced by the lanthanides (Ln) used for doping. While different techniques are able to elucidate the distribution of dopants in the host matrix on different length scale, it turns out that it is not easy to “prove” doping homogeneity. Instead the usual approach is to disprove heterogeneity on different length scales. Analytical methods can establish heterogeneity in different ways: X-ray diffraction (XRD) as a bulk technique can be used to establish heterogeneity by deviation of the lattice parameters from Vegard's rule10 or by the appearance of a non-statistical pair distribution function11 given that the scattering contrast is sufficient. X-ray photoelectron spectroscopy (XPS) offers information about the dopant distribution at the surface on the nm scale.12 Methods based on electron microscopy can prove heterogeneity at a specific location in the sample but not in the bulk. For example, transmission electron microscopy can provide line-scans showing a dopant distribution profile on the nm scale for thin sample layers.13 Energy dispersive X-ray (EDX) mapping in a scanning electron microscope (SEM) can show heterogeneous dopant distributions on the sub-μm scale14,15 at a sample surface to a depth of several μm, depending on the accelerator voltage13 (see below). EDX is considered to be a semi-quantitative analysis technique, as the number of detected X-rays depends on the accelerating voltage, the sample composition, the detection angle and the sample morphology, with especially the latter effect not being easy to correct for. Cathodoluminescence (CL) imaging evaluates the spatial and spectral distribution of the emitted light after excitation by the electron beam. For a certain fixed position of the electron beam, light is generated in a similar volume compared to the characteristic X-rays. The advantage over EDX is an increased sensitivity, allowing to study lower impurity concentrations. Finally, the luminescence characteristics of many impurities depend on the local environment. Hence, the shape of the CL emission spectrum can be used to probe the local chemical composition.16,17 Electron paramagnetic resonance (EPR) studies the signals from paramagnetic dopants in low concentration and extracts information on distribution as well as local environment18 from spectroscopic parameters such as the lineshape.19 A broad linewidth is typical for strong couplings and indicative of heterogeneity.20
In this contribution solid-state NMR is used to investigate co-doping heterogeneity. In case of a diamagnetic solid host with NMR-active nuclei solid-state NMR has been used to investigate the dopant distribution of a single kind of paramagnetic dopant (“mono-doped”) in different ways: spin–lattice T1 relaxation,21–23 line width24–26 and visibility function (see below).27,28 A T1–T2 correlation map was also shown to be useful in studying a paramagnetic site distribution at the surface of porous materials.29 These NMR observables of the host-material are influenced by the hyperfine interaction30,31 between the unpaired electrons of a paramagnetic dopant and the NMR-active nucleus in a non-trivial way in general. For Ln(III) dopants the valence electrons are situated in f-orbitals for which the spin–orbit (SO) interactions dominate over ligand field effects as compared to transition metal atoms for which the order is reversed.32 Thus the magnetism of Ln(III) ions can be predicted neglecting ligand field effects in the high-temperature regime with the exception of Sm(III) and Eu(III). In fact the NMR visibility function, the relaxation behavior and the line-width of host signals have been shown to correlate in a simple way with an increasing Ln(III) doping concentration for the host chosen here.26 Here we make use of the NMR visibility, which is the molar peak area of NMR signal of a paramagnetically doped compound normalized by that of the undoped host. The visibility function is the NMR visibility as a function of doping concentration that can be approximated analytically by a decaying exponential function, if it is assumed that around every paramagnetic dopant a certain volume exists within which NMR nuclei become “invisible” to the NMR experiment. Invisible here means that signals decay very fast so that they do not contribute to the FID which is sampled after the spectrometer deadtime. This concept was independently introduced by different groups in the context of dynamic nuclear polarization33 and for the purpose of studying doping homogeneity.27 It could be shown that in a homogeneous system the visible percentage of the host NMR signal decays in an exponential fashion with increasing doping concentration which can be used to estimate the radius of the blind sphere around a paramagnetic center (formula see experimental part).26,27 In mono-doped materials deviations from this exponential function may serve as a criterion of doping heterogeneity in the above sense on the length scale of the blind-sphere radius26–28 which typically takes values up to ∼2 nm. While in general it may not always be trivial to explain what contributes to the blind sphere radius it appears that for Ln(III) dopants the pseudo-contact shielding is strongly correlated with the size of the blind sphere.26 Correspondingly the blind-spheres for different NMR active nuclei in the same material are related to the size of the gyromagnetic ratio.26 The only known exception for Ln(III) dopants is Gd(III) which is known for its very special relaxation behavior which also makes it an effective contrast agent in magnetic resonance imaging.31
Given the interest in co-doped luminescent materials the target of this study is to investigate whether the NMR visibility function can be extended to co-doped systems and to compare this approach to other commonly used techniques to investigate doping homogeneity.
Experimental
Reference materials for co-doped monazite LaPO4 were obtained with a co-precipitation method and subsequent annealing which previously had been shown to produce homogeneously mono-doped samples.26,28 The starting reagents, which include La2O3 (ChemPur, 99.99%) and dopant materials Gd2O3 (smart elements, 99.99%), Dy2O3 (smart elements, 99.999%), Ho2O3 (smart elements, 99.99%), Yb2O3 (smart elements, 99.99%) and Nd2O3 (ChemPur, 99.99%), were mixed and dissolved in the required ratios in concentrated nitric acid. Upon adding NH4H2PO4 (VWR chemicals) solution in excess a precipitate is formed, washed with water and ethanol, then centrifuged and dried overnight. Annealing is done in corundum crucibles at 1000 °C for 4 h.Solid state NMR measurements were performed on a Bruker Avance II spectrometer at 7.05 T. Magic angle spinning (MAS) was done with 4 mm pencil rotors at 10 kHz with a home-built McKay probe head and a dead-time delay of 15 μs. External quantification of the NMR signals was assisted with a micro-balance (Sartorius MC5). The NMR visibility f was calculated as the observed peak area of the 31P NMR signal per mole of the sample normalized by the peak area per mole of non-doped diamagnetic host. Deconvolution of NMR peaks was done with the program deconv2Dxy.34 The NMR visibility fitting function27,28 for a homogeneously mono-doped sample is defined as 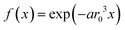 , where r0 is the blind-sphere radius, a is a parameter related to the number density of dopable sites (a = 4/3πNhostUC/VUC), NhostUC is the number of “dopable” sites in the unit cell, x is the fraction of atoms substituted by a paramagnetic dopant (see example) and VUC is the volume of the unit cell. For example, for monazite35 doped with Dy, i.e. LaPO4:Dy or La1−xDyxPO4, a is 0.055 Å−3.
, where r0 is the blind-sphere radius, a is a parameter related to the number density of dopable sites (a = 4/3πNhostUC/VUC), NhostUC is the number of “dopable” sites in the unit cell, x is the fraction of atoms substituted by a paramagnetic dopant (see example) and VUC is the volume of the unit cell. For example, for monazite35 doped with Dy, i.e. LaPO4:Dy or La1−xDyxPO4, a is 0.055 Å−3.
Scanning electron microscopy images were obtained in back-scattered electron detection mode (Hitachi S-3400 N). For elemental mapping with EDX, the connected ThermoScientific Noran System 7 was used. CL radiation emitted by the sample was collected for a 256-by-184 grid (dwell time of 100 ms per point) with an optical fiber and delivered to an Acton SP2300 monochromator and ProEM 1600EMCCDcamera (Princeton Instruments). CL spectra and intensities were processed off-line. All spectra were measured at room temperature and with an accelerating voltage of 20 kV. To avoid electrical charging of the sample, and the associated image deformations, a low background pressure of 20 Pa was used. For EDX quantification of the lanthanide ion concentrations, the L lines were monitored (La: 4.7 keV, Nd: 5.2 keV, Tm: 7.2 keV).
Results and discussion
To investigate the effects of co-doping on the NMR visibility function, reference compounds are required which are homogeneously doped. To this end LaPO4 was co-doped with Nd/Tm, Gd/Dy and Nd/Ho at different concentrations.The synthesis recipe had been used before26,28 to produce single-doped monazite which proved to be homogeneous according to XRD and NMR with respect to the Vegard behavior of the lattice parameters and the NMR visibility function,27 respectively. In the following the doping homogeneity of co-doped LaPO4 is first tested with energy dispersive X-ray mappings and cathodoluminescence. Finally it is shown how the NMR visibility function needs to be modified for application to co-doped materials.
1. Doping homogeneity according to EDX
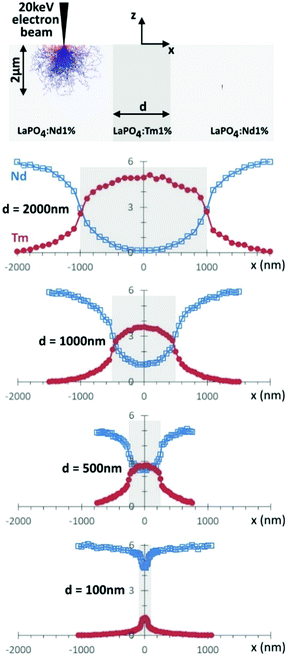
Monazite LaPO4 is obtained by dehydration of rhabdophane LaPO4·H2O which in turn is obtained by precipitation from a solution containing lanthanum and monophosphate ions. Depending on washing steps and how fast the precipitation is conducted, impurities by hydrogen phosphates can be observed which after high temperature annealing convert into LaP3O9.36 This impurity (less than 5% based on NMR peak area quantification results) should not have an influence on the distribution of the lanthanide dopants but may cause the formation of grain boundaries and could give rise to deviations in electron microscopy which is sensitive to the surface of the particles. Powder X-ray diffraction (not shown) indicates a very small impurity of LaP3O9 which is consistent with 31P MAS NMR. Other side phases could not be observed. Nevertheless, given that solubility limits for a solid solution of LaPO4:Ln exist37 and that light lanthanides prefer to crystallize in the monazite structure type but heavy lanthanides prefer to crystallize in the zircon structure type, it is reasonable to further investigate the distribution of lanthanide dopants.Energy dispersive X-ray spectroscopy (EDX) in comparison to XRD is a local technique which elucidates sample composition by the emitted X-ray spectrum inside the excitation volume of the electron beam, which at 20 keV amounts to a 1–2 micrometers in LaPO4. In electron micrographs and EDX maps (Fig. 1 and Fig. S1–S3, ESI†) grain boundaries with different composition in terms of the Ln/P ratio could be observed but apparently the total amount was too small to have an impact on the NMR experiments (see below). The element composition ratios for the different lanthanides reflect the batch composition of the starting materials and are consistent with a homogeneous distribution throughout the material, at the sub-micron length scale. To assess the spatial resolution which could potentially be obtained in the studied material system, Monte Carlo simulations using Casino38,39 have been performed, where the interactions of the incoming electrons with the sample are simulated, including the creation and eventual detection of characteristic X-rays. The chosen electron energy was 20 keV, as a compromise between EDX signal intensity and spatial resolution. Indeed, lowering the electron beam energy reduces the interaction volume, but simultaneously reduces the number of generated X-rays. Specifically for the studied LaPO4:Ln system, the L-lines of the lanthanides are monitored, in order to avoid the strongly overlapping M-lines, which require an electron beam energy of at least 15 keV to have sufficient yield. Given the low concentrations (and thus also the corresponding EDX signal intensities) of the dopants with respect to the La present in the host, this is an experimental constraint. Consequently, it is not obvious what spatial resolution can be obtained if one wants to assess the dopant homogeneity in the co-doped LaPO4 samples. Therefore, La1−x(Nd,Tm)xPO4 was simulated as a model system (Fig. 2), where the doping was considered fully inhomogeneous.
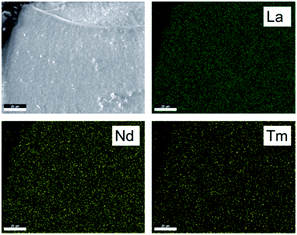 | ||
| Fig. 1 Electron micrographs detected via backscattered electrons (top left) and EDX mappings (top right and bottom) of La0.8Nd0.1Tm0.1PO4. The acceleration voltage is 30 kV. | ||
A slab of La0.99Tm0.01PO4 was inserted in between two slabs consisting of La0.99Nd0.01PO4. The width of the central Tm doped slab was varied from 100 nm to 2 μm, while the (vertical) thickness was taken larger than the penetration depth of the electron beam. Then the electron beam was swept over the sample surface, across the three slabs. For each simulated point of incidence, the obtained EDX signal intensities for Nd and Tm were stored and plotted (Fig. 2). While the lanthanide concentrations follow a step like function, the concentration profile is smoothed by the interaction volume in the sample. For the widest central slab of 2 μm, the concentration profiles show that distinct regions can be found, where e.g. no X-rays originating from Nd are found. For narrower central slabs, a further smoothing occurs, and already at a central width of 1 μm, Nd is measured along the entire profile. When the central Tm-doped slab is only 100 nm wide, the Nd signal only drops by about 20% in the middle of the Tm-doped slab, as compared to positions far away from the central slab. Taking into account the experimental constraints, where the Nd EDX signal will be low given the low doping concentration, the noise on each measured point will likely obscure this small dip in the concentration profile. Similarly, while there is for the central Tm-doped slab of 100 nm a clear increase in the Tm concentration when crossing this slab, the signal intensities will be fairly low (e.g. as when compared to the 2 μm wide slab), because the electrons spread laterally out into the Nd-doped slabs. Consequently, mapping of the dopant concentration by EDX will – under the mentioned constraints of signal intensity – not allow to quantitatively assess the dopant homogeneity on the submicron, or smaller, scale. Of course, if the (vertical) thickness of the sample can be reduced by preparing a thin slab, the spatial resolution increases, as the electrons leave the sample on the bottom side before they can spread too much laterally.
2. Fluorescence spectroscopy
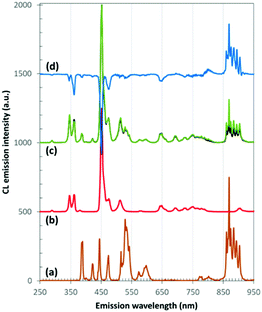
When the electron beam hits the sample, also cathodoluminescent radiation is generated in a similar volume as the characteristic X-rays, used for the EDX analysis. The advantage is however that the CL emission can be strong, translating in a high signal intensity, and that the contributions in the emission spectrum can be related to specific luminescent ions, especially in the case of the 4f–4f emitting trivalent lanthanides. In principle, the emission intensity can be related to the local dopant concentration as there is a linear relation between both, until concentration quenching sets in, where the CL intensity increases sublinearly (or even reduces) for increasing dopant concentration.The CL emission spectra of codoped La0.984Nd0.008Tm0.008PO4 and La0.968Nd0.016Tm0.016PO4, together with those for single doped La0.995Nd0.005PO4 and La0.995Tm0.005PO4, have been measured and compared (Fig. 3). Those spectra were acquired while the electron beam was continuously swept over a large area (100 μm × 100 μm) to find the average emission spectrum. The emission of La0.995Nd0.005PO4 is dominated by the transitions in the infrared (with the 4F3/2 → 4I9/2 transitions around 900 nm), accompanied by several transitions in the visible part of the spectrum, originating from the higher-lying 2F(2)5/2, 4D3/2 and 2P3/2 levels.40 For La0.995Tm0.005PO4, the main emission bands are around 345 nm (3P0 → 3F4), 360 nm (1D2 → 3H6), 455 nm (3P0 → 3H4, 1D2 → 3F4), 475 nm (1G4 → 3H6), 650 nm (1G4 →3F4) and from 700 to 800 nm (3F2, 3F3, 3H4 → 3H6).41 No emission peaks related to other lanthanides were found, pointing at good purity of the prepared phosphors. The CL emission spectrum of the Nd, Tm co-doped La0.984Nd0.008Tm0.008PO4 is essentially the sum of the contributions of both Tm3+ and Nd3+ ions (Fig. 3c), although subtle differences can be spotted as function of the doping concentration, e.g. in the relative intensity of the Tm3+ transitions at 345 nm and 360 nm which originate from 3P0 and 1D2, respectively. To assess the degree of energy transfer between Tm3+ and Nd3+, the dopant concentration of both Tm and Nd was doubled (i.e. the phosphor with composition La0.968Nd0.016Tm0.016PO4, Fig. 3c). This doesn’t dramatically alter the emission spectrum and to better appreciate the differences, the difference spectrum between both co-doped samples is shown in Fig. 3d.
For La0.968Nd0.016Tm0.016PO4, the Nd3+ transitions in the near IR between 850 and 920 nm increase in relative intensity, while the Nd3+ transitions in the visible are slightly reduced, compared to La0.984Nd0.008Tm0.008PO4. For the Tm3+ emission lines, most of the transitions below 675 nm reduce in intensity, where especially the transitions originating from 1D2 reduce in intensity, which could be due to increased cross-relaxation in Tm3+ or to increased energy transfer to Nd3+, as the Tm3+ (1D2) level is at approximately the same energy as the Nd3+ (4D3/2) level with respect to their ground states.
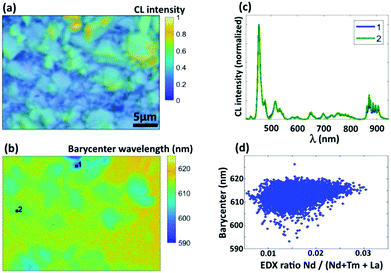
Of course, the CL spectra discussed above are only average CL spectra. Therefore, SEM-CL-EDX mapping was used to probe the distribution of both dopant ions on the submicron scale, where for every pixel the full emission spectrum was recorded in the range from 400 to 920 nm. To assess the doping homogeneity, the barycenter emission wavelength λbc
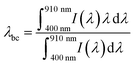 | (1) |
3. NMR: visibility function for paramagnetic co-doping of a diamagnetic host
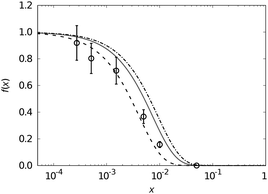
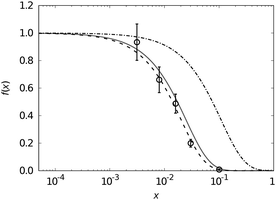
For mono-doped systems, the NMR visibility f(x) for homogeneous doping shows an exponential dependence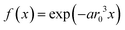 on the doping concentration x, the number density parameter a and the radius of the blind sphere r0. Heterogeneity causes a deviation from the homogeneous curve into the direction of complete phase segregation.28 The curve for complete phase segregation can be estimated assuming a minimum blind sphere radius, which can be illustrated on the example of LaPO4:Tm, i.e. La1−xTmxPO4. Given the mixture falls apart into (1 − x) LaPO4 and x TmPO4 and the NMR active nuclei in TmPO4 are “invisible” because the blind sphere is covering them, then the maximum possible visibility fmax(x) is approaching fmax(x) = 1 − x.27
on the doping concentration x, the number density parameter a and the radius of the blind sphere r0. Heterogeneity causes a deviation from the homogeneous curve into the direction of complete phase segregation.28 The curve for complete phase segregation can be estimated assuming a minimum blind sphere radius, which can be illustrated on the example of LaPO4:Tm, i.e. La1−xTmxPO4. Given the mixture falls apart into (1 − x) LaPO4 and x TmPO4 and the NMR active nuclei in TmPO4 are “invisible” because the blind sphere is covering them, then the maximum possible visibility fmax(x) is approaching fmax(x) = 1 − x.27
The exponential visibility function for mono-doped systems was deduced in two different ways for a homogeneous doping scenario. We derived it by an inductive argument, i.e. a Taylor expansion for the low-doping regime which allowed to parameterize a decaying exponential function and a numerical verification on discrete, crystalline, statistically doped structures27 by counting the numbers of atoms in- and outside the blind-spheres, which also allows to qualify when the assumption of a constant dopant number density is not valid anymore. Griffin and coworkers (ref. 33, Supp. Info. p. S10), independently derived it by an elegant probabilistic ansatz.42 In the latter the idea is to calculate the probability for not finding a small voxel inside any blind-sphere in a given volume V, where the number density n is the number density of blind spheres. In a given volume V in total N = nV blind spheres are introduced.
 | (2) |
It is straightforward to extend this approach to a homogeneous co-doping situation with the dopants Q and P.
 | (3) |
| f(x,y) = exp(−ar13x)exp(−ar23y) | (4) |
| a = 4/3πNUC/VUC | (5) |
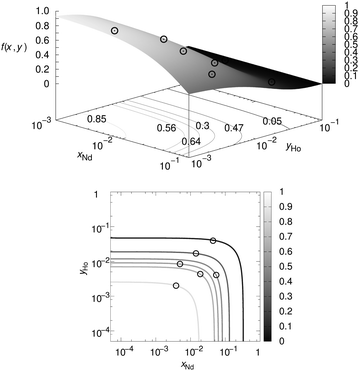
4. Validation of the NMR visibility function for a co-doping scenario
In the next step this model is to be tested experimentally. For this purpose three co-doped sample series have been prepared: La1−x−yGdxDyyPO4, La1−x−yNdxTmyPO4, and La1−x−yNdxHoyPO4. Given the approximation works it should be possible to predict the 3D visibility function f(x,y) from the blind-sphere radii of Gd(III), Dy(III), Nd(III), Tm(III), Ho(III) obtained from mono-doped systems for the homogeneous case, which in a previous study26 were estimated to be 13.5, 12.5, 5.5, 9.0 and 10.5 Å, respectively.The co-doping systems were chosen for three different reasons: first luminescence should be feasible to have another method to disprove homogeneity (see above), second the blind-spheres should have a different origin, i.e. relaxation based quenching of the NMR signal leading to homogeneous broadening, as in the case of Gd(III) and inhomogeneous line-broadening as in the case of Nd(III), Dy(III), Tm(III) and Ho(III),26 and third the radii should differ in size.
To ease the visualization of the NMR visibility f(x,y) the doping concentration of both dopants was increased by an equal amount.
| f(x) = exp(−ar13x)exp(−ar23x) = exp[−a(r13 + r23)x] | (6) |
A point which can be learned from the NMR visibility function f(x,y) is that the sensitivity towards the doping concentrations x and y are scaled by the cubic radius of the corresponding dopants, which means that in case of a significant difference in radius like for Nd(III) and Tm(III) the bigger ion will dominate the visibility curve.
For this reason it makes sense to also test the visibility function f(x,y) without imposing a linear dependence of x and y. Such a 3D plot, the “NMR visibility map”, is shown for La1−x−yNdxHoyPO4 in Fig. 7, where the contour-levels have be chosen according to the expected value for f(x,y) at the point (x,y). Again the experimental data showed good agreement with the assumed model. For the La1−x−yGdxDyyPO4 and La1−x−yNdxTmyPO4 series they are shown in the ESI† (Fig. S4 and S5).
Which advantages and disadvantages does the NMR visibility approach have in comparison to other techniques?
An advantage is that the NMR visibility tests doping homogeneity on a length scale which is of the same order of magnitude as the critical energy transfer distance, which indicates it could have relevance to applications in luminescence.
The NMR visibility function is not directly sensitive to the distribution of the two co-dopants relative to each other on the other hand. For two co-dopants with similar blind-sphere radius, it is possible that the NMR visibility of a system where the co-dopants do not mix, looks the same as a homogeneously co-doped system. The proposed formula provides a simple criterion to test doping homogeneity in co-doped systems, nevertheless.
Conclusions
Doping homogeneity has been evaluated from Å to μm scales by a combination of different techniques: solid-state NMR, EDX and SEM-CL. LaPO4 doped with Tm3+ and/or Nd3+ was studied as a model system. For the co-doped samples, the doping homogeneity was very high at the submicron scale, as witnessed by the almost invariable emission spectrum over the evaluated sample area. It could be shown that the NMR visibility function can be predicted in case of homogeneous doping for co-doped systems given that the blind-sphere radii do not influence each other. This approximation seems to be valid in case of lanthanide dopants Ln(III). The NMR visibility function may thus be used to study doping homogeneity in co-doped systems to achieve higher light yields. The study shows that it is often necessary to apply different techniques to study doping homogeneity: bulk techniques like NMR have the advantage that a deviation from an expected ideal curve can be qualified in a simple number, while microscopic techniques with local resolution power may give qualitative evidence for heterogeneous distributions of dopants.Conflicts of interest
There are no conflicts to declare.References
- J. Zhang, K. Tse, M. Wong, Y. Zhang and J. Zhu, A brief review of co-doping, Front. Phys., 2016, 11, 117405 CrossRef.
- H. Kanno, Y. Hamada and H. Takahashi, Development of OLED with high stability and luminance efficiency by co-doping methods for full color displays, IEEE J. Sel. Top. Quantum Electron., 2004, 10, 30–36 CrossRef CAS.
- H. S. Jang, W. B. Im, D. C. Lee, D. Y. Jeon and S. S. Kim, Enhancement of red spectral emission intensity of Y3Al5O12:Ce3+ phosphor via Pr co-doping and Tb substitution for the application to white LEDs, J. Lumin., 2007, 126, 371–377 CrossRef CAS.
- K. Arai, H. Namikawa, K. Kumata, T. Honda, Y. Ishii and T. Handa, Aluminum or phosphorus co-doping effects on the fluorescence and structural properties of neodymium-doped silica glass, J. Appl. Phys., 1986, 59, 3430–3436 CrossRef CAS.
- G. Blasse, Energy transfer in oxidic phosphors, Phys. Lett. A, 1968, 28, 444–445 CrossRef CAS.
- G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer-Verlag, Berlin Heidelberg, 1994 Search PubMed.
- K. Van den Eeckhout, P. F. Smet and D. Poelman, Persistent Luminescence in Eu2+-Doped Compounds: A Review, Materials, 2010, 3, 2536–2566 CrossRef CAS.
- P. F. Smet, K. Van den Eeckhout, O. Q. De Clercq and D. Poelman, Persistent Phosphors, Handbook on the Physics and Chemistry of Rare Earths, including Actinides, Elsevier, 2015, ch. 274, vol. 48, pp. 1–108 Search PubMed.
- W. Horwitz, Nomenclature for sampling in analytical chemistry (Recommendations 1990), Pure Appl. Chem., 1990, 62, 1193–1208 CAS.
- L. Vegard, Die Konstitution der Mischkristalle und die Raumfüllung der Atome, Z. Phys., 1921, 5, 17–26 CrossRef CAS.
- S. J. L. Billinge, Th. Proffen, V. Petkov, J. L. Sarrao and S. Kycia, Evidence for charge localization in the ferromagnetic phase of La1-xCaxMnO3 from high real-space-resolution x-ray diffraction, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 1203–1211 CrossRef CAS.
- T. Susi, T. Pichler and P. Ayala, X-ray photoelectron spectroscopy of graphitic carbon nanomaterials doped with heteroatoms, Beilstein J. Nanotechnol., 2015, 6, 177–192 CrossRef PubMed.
- F. Sidiroglou, A. Roberts and G. Baxter, Contributed Review: A review of the investigation of rare-earth dopant profiles in optical fibers, Rev. Sci. Instrum., 2016, 87, 041501 CrossRef CAS PubMed.
- G. Servanton, R. Pantel, M. Juhel and F. Bertin, Two-dimensional quantitative mapping of arsenic in nanometer-scale silicon devices using STEM EELS–EDX spectroscopy, Micron, 2009, 40, 543–551 CrossRef CAS PubMed.
- K. Van den Eeckhout, P. F. Smet and D. Poelman, Persistent luminescence in rare-earth codoped Ca2Si5N8:Eu2+, J. Lumin., 2009, 129, 1140–1143 CrossRef CAS.
- T. Coenen and N. M. Haegel, Cathodoluminescence for the 21st century: Learning more from light, Appl. Phys. Rev., 2017, 4, 031103 Search PubMed.
- D. Poelman and P. F. Smet, Time resolved microscopic cathodoluminescence spectroscopy for phosphor research, Phys. B, 2014, 439, 35–40 CrossRef CAS.
- D. Ghica, I. D. Vlaicu, M. Stefan, V. A. Maraloiu, A. C. Joita and C. Ghica, Tailoring the Dopant Distribution in ZnO:Mn Nanocrystals, Sci. Rep., 2019, 9, 6894 CrossRef PubMed.
- H. Deters, J. F. de Lima, C. J. Magon, A. S. S. de Camargo and H. Eckert, Structural models for yttrium aluminium borate laser glasses: NMR and EPR studies of the system (Y2O3)0.2–(Al2O3)x–(B2O3)0.8−x, Phys. Chem. Chem. Phys., 2011, 13, 16071–16083 RSC.
- N. Pathak, S. K. Gupta, P. S. Ghosh, A. Arya, V. Natarajan and R. M. Kadam, Probing local site environments and distribution of manganese in SrZrO3:Mn; PL and EPR spectroscopy complimented by DFT calculations, RSC Adv., 2015, 5, 17501–17513 RSC.
- T. Harazono, R. Adachi, N. Kijima and T. Watanabe, 89Y MAS NMR in Red Phosphor, Eu-Doped Y2O2S. Assignment of Peaks Shifted by Paramagnetic Eu3+, Spin Lattice Relaxation Time, and Eu Distribution, Bull. Chem. Soc. Jpn., 1999, 72, 2655–2664 CrossRef CAS.
- S. Maron, G. Dantelle, T. Gacoin and F. Devreux, NMR and ESR relaxation in Nd- and Gd-doped LaPO4: towards the accurate determination of the doping concentration, Phys. Chem. Chem. Phys., 2014, 16, 18788–18798 RSC.
- S. Maron, N. Ollier, T. Gacoin and G. Dantelle, Determination of paramagnetic concentrations inside a diamagnetic matrix using solid-state NMR, Phys. Chem. Chem. Phys., 2017, 19, 12175–12184 RSC.
- T. Harazono, E. Yokota, H. Uchida and T. Watanabe, 89Y-Static and MAS NMR, and 27AlMAS NMR in Green Phosphor, Tb-Doped Y3Al5O12 and Luminous Characteristics, Bull. Chem. Soc. Jpn., 1998, 71, 2797–2805 CrossRef CAS.
- T. Harazono, E. Yokota, H. Uchida and T. Watanabe, Luminous Characteristics and 89Y-Static NMR in Red Phosphor, Eu-Doped Y2O3, Bull. Chem. Soc. Jpn., 1998, 71, 825–829 CrossRef CAS.
- W. Li, Q. Zhang, J. J. Joos, P. F. Smet and J. Schmedt auf der Günne, Blind spheres of paramagnetic dopants in solid state NMR, Phys. Chem. Chem. Phys., 2019, 21, 10185–10194 RSC.
- W. Li, V. R. Celinski, J. Weber, N. Kunkel, H. Kohlmann and J. Schmedt auf der Günne, Homogeneity of doping with paramagnetic ions by NMR, Phys. Chem. Chem. Phys., 2016, 18, 9752–9757 RSC.
- W. Li, M. Adlung, Q. Zhang, C. Wickleder and J. Schmedt auf der Günne, A guide to brighter phosphors – linking luminescence properties to doping homogeneity probed by NMR, ChemPhysChem, 2019, 20, 3245–3250 CrossRef CAS PubMed.
- C. D’Agostino and P. Bräuer, Exploiting enhanced paramagnetic NMR relaxation for monitoring catalyst preparation using T1–T2 NMR correlation maps, React. Chem. Eng., 2019, 4, 268–272 RSC.
- A. J. Pell, G. Pintacuda and C. P. Grey, Paramagnetic NMR in solution and the solid state, Prog. Nucl. Magn. Reson. Spectrosc., 2019, 111, 1–271 CrossRef CAS PubMed.
- I. Bertini, C. Luchinat, G. Parigi and E. Ravera, NMR of Paramagnetic Molecules, 2nd edn, Elsevier, Boston, 2017 Search PubMed.
- H. Lueken, Magnetochemie, ed. B. G. Teubner, Stuttgart/Leipzig, 1999 Search PubMed.
- B. Corzilius, L. B. Andreas, A. A. Smith, Q. Z. Ni and R. G. Griffin, Paramagnet induced signal quenching in MAS–DNP experiments in frozen homogeneous solutions, J. Magn. Reson., 2014, 240, 113–123 CrossRef CAS PubMed.
- D. Jardón-Álvarez and J. Schmedt auf der Günne, Reduction of the temperature gradients in laser assisted high temperature MAS NMR, Solid State Nucl. Magn. Reson., 2018, 94, 26–30 CrossRef PubMed.
- Y. Ni, J. M. Hughes and A. N. Mariano, Crystal chemistry of the monazite and xenotime structures, Am. Mineral., 1995, 80, 21–26 CAS.
- C. Penot, E. Champion and P. Goursat, Synthesis and Characterisation of Lanthanum Phosphate Powders, Phosphorus Res. Bull., 1999, 10, 307–312 CrossRef CAS.
- N. Clavier, R. Podor and N. Dacheux, Crystal chemistry of the monazite structure, J. Eur. Ceram. Soc., 2011, 31, 941–976 CrossRef CAS.
- D. Drouin, A. R. Couture, D. Joly, X. Tastet, V. Aimez and R. Gauvin, CASINO V2.42—A Fast and Easy-to-use Modeling Tool for Scanning Electron Microscopy and Microanalysis Users, Scanning, 2007, 29, 92–101 CrossRef CAS PubMed.
- H. Demers, N. Poirier-Demers, A. R. Couture, D. Joly, M. Guilmain, N. de Jonge and D. Drouin, Three-dimensional electron microscopy simulation with the CASINO Monte Carlo software, Scanning, 2011, 33, 135–146 CrossRef CAS PubMed.
- M. F. Joubert, B. Jacquier, C. Linares and R. M. Macfarlane, Relaxation of the high lying states and coordination numbers of Nd3+ ions in fluorides, J. Lumin., 1992, 53, 477–482 CrossRef CAS.
- M. Quintanilla, N. O. Núñez, E. Cantelar, M. Ocaña and F. Cussó, Tuning from blue to magenta the up-converted emissions of YF3:Tm3+/Yb3+ nanocrystals, Nanoscale, 2011, 3, 1046–1052 RSC.
- H. L. Weissberg, Effective Diffusion Coefficient in Porous Media, J. Appl. Phys., 1963, 34, 2636–2639 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cp05599a |
| This journal is © the Owner Societies 2020 |