Free volume, gas permeation, and proton conductivity in MIL-101-SO3H/Nafion composite membranes†
Abstract
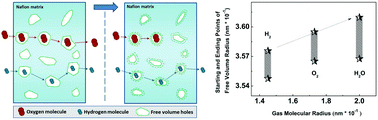
A series of MIL-101-SO3H/Nafion composite membranes was synthesized. They show an improved proton conductivity, due to the abundance of SO3H groups, which fosters proton conduction by binding the water molecules and enabling a larger number of conducting sites. Gas (including water vapor, hydrogen, and oxygen) permeability, crystallinity, and free volumes of the MIL-101-SO3H/Nafion composite membranes were investigated, as well as their correlation. By increasing the MIL-101-SO3H content, the gas permeability of the membranes significantly decreases, since the crystalline region is larger and the water-bearing MIL-101-SO3H particles are efficient barriers for the gas molecules. The gas permeation in the composite membranes is a very complex process and the results indicate no simple linear relation between the gas permeability and the free volume size (VFV), or between the gas permeability and the crystallinity. Moreover, it is very interesting to observe that the influence of VFV on the gas permeability is closely related to the size of the particular gas molecules: the larger the size of the gas molecules, the larger the free volume needed to achieve their rapid diffusion in the membrane. The results suggest the presence of a threshold value for VFV, which depends on the size of the gas molecules: when VFV is lower than this value, the gas molecules cannot easily jump through neighboring free volumes to a neighboring site, and, as a result, the permeability drops quickly.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...