Revealing the thermodynamics of individual catalytic steps based on temperature-dependent single-particle nanocatalysis†
Abstract
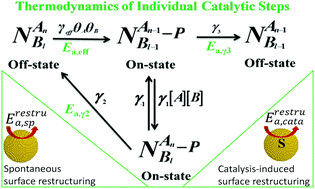
Due to the intrinsic heterogeneity of nanocatalysis, many underlying catalytic details on nanocatalysts are hidden in ensemble-averaged measurements. Here, the single-molecule approach was adopted to study the temperature-dependent catalytic kinetics and dynamics of individual Pt nanoparticles and then reveal the thermodynamics of individual catalytic steps on Pt nanoparticles. In this way, the temperature-dependent catalytic kinetics (the effective rate constant of the product formation process, the rate constants of the direct/indirect production desorption process and the substrate adsorption equilibrium constants) and thermodynamics (free energy, entropy and enthalpy of substrate adsorption) were obtained systematically at the single particle level. Based on such results, we further obtained the activation energies of the catalytic product formation step and the direct/indirect product desorption steps. Moreover, by analyzing the temperature-dependent surface restructuring rates of individual Pt nanocatalysts, the activation energies of both the catalysis-induced surface restructuring and the spontaneous surface restructuring were obtained for the first time. All these results obtained here deepen our understanding of the catalytic thermodynamics of nanocatalysts.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...