Structure, stability and water adsorption on ultra-thin TiO2 supported on TiN†
Abstract
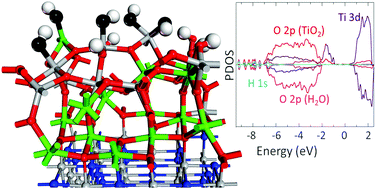
Interfacial metal-oxide systems with ultra-thin oxide layers are of high interest for their use in catalysis. The chemical activity of ultra-thin metal-oxide layers can be substantially enhanced compared to interfacial models with thicker oxide. In this study, we present a Density Functional Theory (DFT) investigation of the structure of ultra-thin rutile layers (one and two TiO2 layers) supported on TiN and the stability of water on these interfacial structures. The rutile layers are stabilized on the TiN surface through the formation of interfacial Ti–O bonds. Charge transfer from the TiN substrate leads to the formation of reduced Ti3+ cations in TiO2. The concentration of Ti3+ is proportionally higher in the ultra-thin oxide, compared to interfacial models with thicker oxide layers. The structure of the one-layer oxide slab is strongly distorted at the interface while the thicker TiO2 layer preserves the rutile structure. The energy cost for the formation of a single O vacancy in the one-layer oxide slab is only 0.5 eV with respect to the ideal interface. For the two-layer oxide slab, the introduction of several vacancies in an already non-stoichiometric system becomes progressively more favourable, which indicates the stability of the highly defective interfaces. Isolated water molecules dissociate when adsorbed at the TiO2 layers. At higher coverages, the preference is for molecular water adsorption. Our ab initio thermodynamics calculations show the fully water covered stoichiometric models as the most stable structure at typical ambient conditions. This behaviour is similar to that observed on thicker oxide in TiO2–TiN interfaces or pure TiO2 surfaces. In contrast, interfacial models with multiple vacancies are most stable at low (reducing) oxygen chemical potential values. The high concentration on reduced Ti3+ introduces significant distortions in the O-defective slab. Whereas, a water monolayer adsorbs dissociatively on the highly distorted 2-layer TiO1.75–TiN interface, where the Ti3+ states lying above the top of the valence band contribute to a significant reduction of the energy gap compared to the stoichiometric TiO2–TiN model. Our results provide a guide for the design of novel interfacial systems containing ultra-thin TiO2 with potential application as photocatalytic water splitting devices.


 Please wait while we load your content...
Please wait while we load your content...