Structural features of selected protic ionic liquids based on a super-strong base†
Abstract
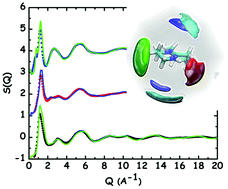
Protic ionic liquids (PIL) were prepared from a super-strong base 1,7-diazabicyclo[5.4.0]undec-7-ene (DBU) and super-strong acids, trifluoromethane sulfonic acid (TfOH), and (trifluoromethanesulfonyl)-(nonafluorobutylsulfonyl)imide, (IM14H), ([DBUH][TfO] and [DBUH][IM14], respectively; the latter for the first time) and their chemical and physical properties and structural features have been explored using a synergy of experimental and computational tools. The short range order in neat DBU, as well as the long range structural correlations induced by charge correlation and hydrogen bonding interactions in the ionic liquids, have been explored under ambient conditions, where these compounds are proposed for a variety of applications. Similar to other [DBUH]-based PILs, the probed ones behave as good ionic liquids. Molecular dynamics-rationalised X-ray diffraction patterns show the major role played by hydrogen bonding in affecting morphology in these systems. Additionally, we find further evidence for the existence of fluorous domains in [DBUH][IM14], thus potentially extending the range of applications for these PILs.


 Please wait while we load your content...
Please wait while we load your content...