Effect of solvent environment on excited state intramolecular proton transfer in 2-(4-(dimethylamino)phenyl)-3-hydroxy-6,7-dimethoxy-4h-chromen-4-one†
Abstract
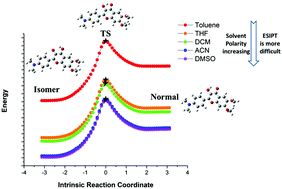
The new ratiometric fluorescent probe 2-(4-(dimethylamino)phenyl)-3-hydroxy-6,7-dimethoxy-4h-chromen-4-one (HOF) monitoring of methanol in biodiesel was discovered experimentally (T. Y. Qin et al., Sens. Actuators, B, 2018, 277, 484–491). But the experimental study did not report the reaction mechanism in detail. In this study, density functional theory (DFT) and time-density functional theory (TDDFT) methods were used to theoretically study the excited-state intramolecular proton transfer (ESIPT) process of the HOF molecule. The molecular structure in the ground state and the excited state was optimized, and the infrared vibrational spectra, the frontier molecular orbitals, the charge transfer, the potential energy curves and the transition-state structures were calculated. The calculated results prove that the solvent polarity has a great influence on the ESIPT reaction of the HOF molecule. As the solvent polarity increased, the intensity of the intramolecular hydrogen bond decreased, and ESIPT was more difficult to occur. This work has studied the mechanism of the ESIPT reaction in more detail, and paved the way for future research on HOF molecules.


 Please wait while we load your content...
Please wait while we load your content...