LuPO4:Yb phosphor with concerted UV and IR thermoluminescent emissions by quantum cutting at high temperatures
Abstract
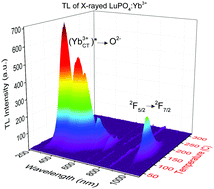
Thermoluminescence of LuPO4:0.1%Yb3+ sintered ceramics was investigated and simultaneous infrared 2F5/2 → 2F7/2 and UV-blue (YbCT3+)* → O2− charge transfer emissions of the Yb3+ impurity were observed around 150 °C (423 K) for the first time. Both photons were generated by one excited Yb3+*. LuPO4:Yb3+ was thus proved to be the first system showing the quantum cutting effect in thermoluminescence. Low concentration of the dopant was proved crucial to observe an intense CT emission at so high temperatures. These data revise deeply those reported previously on the thermal quenching of Yb3+ charge transfer luminescence in orthophosphates. In was formerly claimed that CT luminescence of Yb3+ in LuPO4 and similar hosts is quenched below 300 K. Similarly, the thermoluminescent emission of LuPO4:Yb3+ above room temperature was previously reported to appear only in the IR part of the spectrum around 980 nm. Our results fundamentally change this picture and prove that CT luminescence of Yb3+ in orthophosphates appears to be significant even above 150 °C (423 K). We demonstrate the great significance of the activator concentration in its CT luminescence thermal quenching. The Yb3+ impurity ion was found to act both as an electron trap and as a recombination center. Our data open the possibility to generate intense CT luminescence of Yb3+ in orthophosphates at room temperature and above which may make such phosphors rational for applications previously considered unattainable for them.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...