Computational insight into hydrogen persulfide and a new additive model for chemical and biological simulations†
Abstract
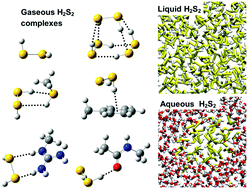
S-Sulfhydration of cysteine to the Cys-SSH persulfide is an oxidative post-translational modification that plays an important regulatory role in many physiological systems. Though hydrogen persulfide (H2S2) has recently been established as a signaling and cellular sulfhydration reagent, the chemistry and chemical biology of persulfides remain poorly explored. We first report an extensive high-level ab initio quantum chemical investigation of (H2S2)n, (H2S2)m·H2O, and (H2O)m·H2S2 clusters (n = 1–3 and m = 1, 2) and of H2S2 complexes with 19 compounds that model the side chains of naturally-occurring amino acids. The high polarizability of S necessitates the use of large, very diffuse, basis sets for proper description of H2S2 and its complexes. H2S2 possesses a skewed equilibrium geometry, with nonpolar trans and more polar cis conformers 6 and 8 kcal mol−1 higher in energy, respectively; the skewed conformation is preserved in all neutral and cationic complexes while a cis geometry prevails in some anionic complexes. H2S2 is found to be a better H-bond donor and a poorer acceptor than H2S, and that in complexes with H2O, alcohols and amines, H2S2 is a better H-bond donor. Radical delocalization on both S atoms stabilizes the perthiyl (HSS˙) over the thiyl (HS˙) radical and results in a ∼20 kcal mol−1 lower S–H homolytic bond dissociation in H2S2, making it a potential antioxidant. A simple additive model is optimized for H2S2 and used together with the TIP3P model and the CHARMM36 all-atom force field (FF) to investigate the structure and thermodynamic properties of liquid H2S2 and the solubility of H2S2 in water, and to model H2S2–protein interactions (for which new FF parameters are further developed). Very weak H-bonding characterizes liquid H2S2 and it is found immiscible in liquid water with a trend in H-bonding strengths between H2S2 and H2O in the order O–H⋯O ≫ S–H⋯O > O–H⋯S. This work does not only provide a thorough description of the structure and energetics of H2S2 and its various complexes, but also yields a reliable FF for investigating H2S2 in chemistry and biology.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...