Tweaking the proton transfer triggered proton transfer of 3,5-bis(2-hydroxyphenyl)-1H-1,2,4-triazole†‡
Abstract
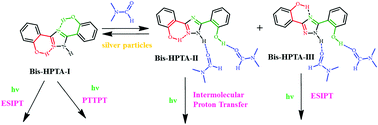
The proton transfer of 3,5-bis(2-hydroxyphenyl)-1H-1,2,4-triazole (bis-HPTA), a fluorophore proficient in self-aided twin proton transfer, has been explored in the presence of dimethylformamide (DMF). The proton transfer is completely altered in the presence of DMF (compared to that in other solvents). Bis-HPTA forms a hydrogen bonded complex with DMF molecules, which shift the conformer equilibrium. Theoretical calculations predicted that the DMF complexes of bis-HPTA-II and bis-HPTA-III are more stable than the complexes of other conformers. Upon excitation, bis-HPTA-II transfers a proton to a DMF molecule to form an anion and bis-HPTA-III transfers a proton to a ring nitrogen to form a keto form. Silver particles reverse the effect of DMF. They regenerate bis-HPTA-I by removing the intermolecular hydrogen bond and thereby they reestablish the proton transfer triggered proton transfer (PTTPT).


 Please wait while we load your content...
Please wait while we load your content...