Stabilization of AgI's polar surfaces by the aqueous environment, and its implications for ice formation†
Abstract
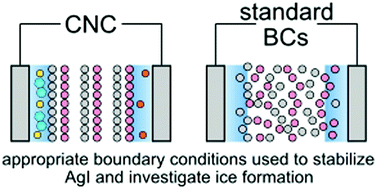
Silver iodide is one of the most potent inorganic ice nucleating particles known, a feature generally attributed to the excellent lattice match between its basal Ag-(0001) and I-(000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) surfaces, and ice. This crystal termination, however, is a type-III polar surface, and its surface energy therefore diverges with crystal size unless a polarity compensation mechanism prevails. In this simulation study, we investigate to what extent the surrounding aqueous environment is able to provide such polarity compensation. On its own, we find that pure H2O is unable to stabilize the AgI crystal in a physically reasonable manner, and that mobile charge carriers such as dissolved ions, are essential. In other words, proximate dissolved ions must be considered an integral part of the heterogeneous ice formation mechanism. The simulations we perform utilize recent advances in simulation methodology in which appropriate electric and electric displacement fields are imposed. A useful by-product of this study is the direct comparison to the commonly used Yeh–Berkowitz method that this enables. Here we find that naive application of the latter leads to physically unreasonable results, and greatly influences the structure of H2O in the contact layer. We therefore expect these results to be of general importance to those studying polar/charged surfaces in aqueous environments.
) surfaces, and ice. This crystal termination, however, is a type-III polar surface, and its surface energy therefore diverges with crystal size unless a polarity compensation mechanism prevails. In this simulation study, we investigate to what extent the surrounding aqueous environment is able to provide such polarity compensation. On its own, we find that pure H2O is unable to stabilize the AgI crystal in a physically reasonable manner, and that mobile charge carriers such as dissolved ions, are essential. In other words, proximate dissolved ions must be considered an integral part of the heterogeneous ice formation mechanism. The simulations we perform utilize recent advances in simulation methodology in which appropriate electric and electric displacement fields are imposed. A useful by-product of this study is the direct comparison to the commonly used Yeh–Berkowitz method that this enables. Here we find that naive application of the latter leads to physically unreasonable results, and greatly influences the structure of H2O in the contact layer. We therefore expect these results to be of general importance to those studying polar/charged surfaces in aqueous environments.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...