Isomer and conformer selective atmospheric pressure chemical ionisation of dimethyl phthalate†
Abstract
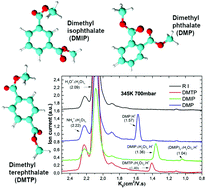
In this work we have studied the ionisation mechanism of Atmospheric Pressure Chemical Ionisation (ACPI) for three isomers of dimethyl phthalate (dimethyl phthalate – DMP (ortho – isomer), dimethyl isophthalate – DMIP (meta) and dimethyl terephthalate – DMTP (para)) using Ion Mobility Spectrometry (IMS) and IMS combined with an orthogonal acceleration Time of Flight Mass Spectrometer (oa-TOF MS). The molecules were chemically ionised using reactant ions H+·(H2O)n (n = 3 and 4). The positive IMS and IMS-oaTOF mass spectra of the isomers showed significant differences in the ion mobilities and in the ion composition. The IMS – oaTOF spectra consisted of clusters of ions M·H+·(H2O)n with different degrees of hydration (n = 0, 1, 2, 3) for different isomers. In the case of the DMP isomer, we have observed almost exclusive formation of M·H+ by proton transfer ionisation, while in the case of DMIP and DMTP, hydrated ions M·H+·(H2O)n (n = 1, 2, 3) and M·H+·(H2O)n (n = 0, 1, 2) respectively were detected, formed via adduct formation reactions. This behaviour was elucidated by differences in ionisation processes. In order to elucidate the ionisation processes we have carried out DFT calculations of the structures and energies of the neutral and protonated and hydrated isomers (for different conformers) and their corresponding proton affinities (PA) and hydration energies.


 Please wait while we load your content...
Please wait while we load your content...