DFT study on the effect of proximal residues on the Mycobacterium tuberculosis catalase-peroxidase (katG) heme compound I intermediate and its bonding interaction with isoniazid†
Abstract
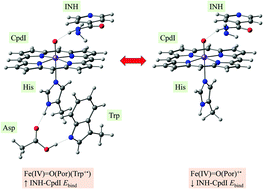
Isoniazid (INH) is converted into isonicotinyl radical through its interaction with the catalase-peroxidase (katG) enzyme present in the cells of Mycobacterium tuberculosis (M. tb.), the bacteria that causes the tuberculosis disease. This process is important because resistance of M. tb. cells to INH treatment has been associated with the failure of completion of this process. However, this process is poorly understood and there are a variety of conflicting theories about the details of the mechanism of this process. One theory suggests that INH binds to katG and transfers a single electron to the heme while the heme is in its two electron oxidized state, compound I [Fe(IV)Por˙+] (CpdI). In this study, DFT calculations at the UB3LYP/6-31g(d)-LANL2DZ level of theory are used to study the M. tb. katG CpdI molecule. Different models of the M. tb. CpdI molecule were prepared and the calculations revealed the impact of Trp321 on the electronic properties of the heme. Without Trp321 the heme assumed a triradical state with single electrons on the πxy and πyz orbitals of Fe and another on the a2u orbital of the porphyrin ring that can either be coupled with the first two, to assume a quartet state, or decoupled to form a doublet state. With Trp321, however, a transfer of an electron from the πTrp orbital to a2u porphyrin orbital leads to loss of radical character of the porphyrin and leaves the Trp321 group with a radical character. INH was observed to have strong interaction with CpdI and the absence of Trp321 significantly decreased the binding energy by 2 kcal mol−1 explaining the importance of Trp321 in the binding of INH. The results in this study show the importance of Trp321 in the binding of INH and its effect on its electronic properties, which is consistent with previous experimental findings that mutation of Trp321 results in an increase in drug resistance.


 Please wait while we load your content...
Please wait while we load your content...