A green solvent for operating highly efficient low-power photon upconversion in air†
Abstract
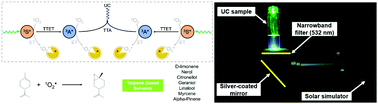
D-Limonene, obtained from the rind of citrus fruits, was demonstrated as a green solvent to realize air-stable and highly efficient triplet–triplet annihilation photon upconversion (TTA-UC). This natural low-toxic compound also contributed to noncoherent UC excited by a solar simulator in air, making TTA-UC materials promising candidates in solar energy and other practical applications. The rapid deoxygenating ability of D-limonene was thoroughly investigated. This system demonstrated very good UC performance for a fluid solution under ambient conditions. Besides, other eight types of terpene were also explored to enrich the alternatives for air-stable TTA-UC in protic and aprotic fluidic environments. This work provides a terpene-based protective platform for oxygen-sensitive TTA-UC applications ranging from life science to photonic devices.


 Please wait while we load your content...
Please wait while we load your content...