A “non-dynamical” way of describing room-temperature paramagnetic manganese oxide†
Sangmoon
Yoon
ab,
Seoung-Hun
Kang‡
b,
Sangmin
Lee
ab,
Kuntae
Kim
a,
Jeong-Pil
Song§
b,
Miyoung
Kim
*a and
Young-Kyun
Kwon
 *b
*b
aDepartment of Materials Science and Engineering, Seoul National University, Seoul, 08826, Korea. E-mail: mkim@snu.ac.kr
bDepartment of Physics and Research Institute for Basic Sciences, Kyung Hee University, Seoul, 02447, Korea. E-mail: ykkwon@khu.ac.kr
First published on 6th May 2019
Abstract
We present a new approach based on static density functional theory (DFT) to describe paramagnetic manganese oxides, representative paramagnetic Mott insulators. We appended spin noncollinearity and a canonical ensemble to the magnetic sampling method (MSM), which is one of the supercell approaches based on the disordered local moment model. The combination of the noncollinear MSM (NCMSM) with DFT+U represents a highly favorable computational method called NCMSM+U to accurately determine the paramagnetic properties of MnO with moderate numerical cost. The effects of electron correlations and spin noncollinearity on the properties of MnO were also investigated. We found that the spin noncollinearity plays an important role in determining the detailed electronic profile and precise energetics of paramagnetic MnO. Our results illustrate that the NCMSM+U approach may be used for insulating materials as an alternative to the ab initio framework of dynamic mean field theory based on DFT in the simulation of the room-temperature paramagnetic properties.
1 Introduction
Manganese oxides have received a lot of attention as they are among the most promising materials for applications in acquisition and storage of sustainable energy, for example, in catalysts, supercapacitors, and lithium-ion batteries. They have the potential to afford various oxidation or reduction reactions as manganese has a multivalent character, and nanosynthesis and surface treatment techniques are also being rapidly developed to support such capabilities. Furthermore, manganese is an earth abundant and eco-friendly element. Thus, attempts are underway to use nano-sized manganese oxides in energy-related devices.1–22 For more effective and efficient development of such devices, it is highly required to investigate systematically and theoretically the room-temperature properties of nanostructured manganese oxides.Manganese oxides are usually paramagnetic Mott insulators under ambient conditions, exhibiting a Mott insulating state despite spin fluctuations because of the strong correlations of d electrons. Thus, for the theoretical study of manganese oxides, it is a primary task to confirm the Mott insulating states. Unfortunately, density functional theory (DFT) calculations fail consistently to describe such Mott insulating states because of their spurious self interactions. In fact, this failure had been one of the central issues in DFT studies of the past decades. Now, it is partially resolved through the Coulomb-interaction corrections of localized electrons such as DFT+U,23,24 hybrid functionals,25,26 GW27 or self-interaction-correction.28 Manganese oxides have also been intensively studied by adopting these approaches.29–36 However, all these extended DFT methods require the materials to be in a spin- or orbital-ordered state, because the electron correlations are still corrected at the static mean field level.
A consistent description of paramagnetic Mott insulators became possible after the development of DFT plus dynamic mean field theory (DFT+DMFT),37,38 where both strong correlations and spin fluctuations are spontaneously involved through the frequency-dependent self-energy. It was, for example, used to understand the paramagnetic Mott insulator to metal transition of MnO.39 Moreover, this method has provided a new direction in the research of many strongly correlated materials, such as heavy fermion systems40,41 and high-temperature superconductors.42,43 However, DFT+DMFT is not suitable for the material design of paramagnetic Mott insulators, because its application to surfaces, interfaces, defects, or various other configurations with large super cells is nearly unfeasible. Meanwhile, disordered local moment (DLM) based DFT+U calculations have also been recently discussed.44 The DLM-based approach involves the effects of spin fluctuations at the static limit. It has successfully described high-temperature properties of some specific correlated systems, for example, several transition-metal oxides and nitrides.44–49 The DLM approach has been implemented in DFT+U in two different manners. One employs the Korringa–Kohn–Rostoker (KKR) Green's function method with the coherent potential approximation (CPA), and the other uses supercells that imitate magnetic disorder. The KKR-CPA method can calculate the electronic structure of disordered systems efficiently, but the expansion beyond the bulk calculation is limited because of the spherical approximation for the one-electron potential. On the other hand, the supercell approach does not have such restrictions, but it generally requires hundreds of atoms to minimize the spurious interactions from the periodic repetition of magnetic disorder. That is, both approaches have advantages and disadvantages. As computing power has increased rapidly and the need for material research has grown, the supercell approach has naturally gained more attention, and related methodologies have been further developed.44–47
The magnetic disorder in a paramagnetic state can be characterized by the spin–spin correlation function, defined as
 | (1) |
Generally, in Mott physics, quasiparticles can emerge with renormalized masses and finite lifetimes, displaying a three-peak spectral structure. However, for an insulating phase, spectral weight transfers from the quasiparticle peak to the Hubbard bands, and thus quasiparticles eventually disappear at the Mott transition and dynamical charge correlations become drastically slow. Hence, it is expected that the DLM-based DFT+U calculation could describe the physical properties of paramagnetic Mott insulators if the effects of magnetic disorder and static self-energies are included adequately. We, therefore, further improved the MSM approach to be accurate and computationally scalable for paramagnetic Mott insulators. Specifically, we revised the MSM approach to consider the spin noncollinearity and to take the canonical ensemble average. We named this approach “noncollinear MSM based on DFT plus U” (NCMSM+U); it is illustrated in Fig. 1. The motivation and importance of the inclusion of spin noncollinearity will be discussed in the results section.
 | ||
| Fig. 1 Schematic illustration of the noncollinear magnetic sampling method (NCMSM) for the paramagnetic Mott insulating phase of MnO. Detailed explanations are given in the text. | ||
To verify the validity and efficiency of our NCMSM+U approach, we selected paramagnetic MnO, which is not only a representative example of paramagnetic manganese oxides where quasiparticles are suppressed, but also an important material for real applications. It has been directly utilized for oxygen evolution reaction catalysts6–8 or for lithium ion batteries.15–17 Thus, the accurate estimation of various room-temperature properties of paramagnetic MnO is an important task for rational device design in real applications. In this paper, we report that the NCMSM+U approach yields various properties of paramagnetic MnO comparable to those calculated from DFT+DMFT. We also investigate the effects of electron correlations and spin noncollinearity on those properties, and discuss the application of our NCMSM+U method to paramagnetic materials with superexchange interactions.
2 Computational details and methodology
We constructed magnetically noncollinearly disordered supercells for NCMSM using two random number generators, which independently determine the azimuthal and polar angle of each magnetic moment. We set the initial magnitude of each magnetic moment to an experimental value of 4.58 μB,50 where μB is the Bohr magneton, with a constraint of the total magnetic moment to be zero (see the ESI†). Although a larger supercell would provide better results, such as the effects of the spin correlations, we used a 2 × 2 × 2 supercell with 64 atoms (32 manganese atoms and 32 oxygen atoms) for NCMSM due to limited computational resources. A series of DFT calculations were conducted using the Vienna ab initio simulation package (VASP) code.51 Perdew–Burke–Ernzerhof plus Hubbard correction (PBE+U) was used for the exchange–correlation functional,52 in which the double-counting interactions were corrected in the fully localized limit (FLL).24,53 Here, it should be discerned that the parameter required in the noncollinear model is the on-site Coulomb interaction U whereas that required in the collinear model is the effective on-site Coulomb interaction Ueff.53 A plane wave basis set with a cutoff energy of 500 eV was used to expand the electronic wave functions, and the valence electrons were described using the projector-augmented wave potentials. The lattice constants in all the cases were fixed to the experimental value of 4.4315 Å50 to prevent the artificial deformation that would be induced by the magnetic disorder. A Γ-centered 4 × 4 × 4 Monkhorst–Pack k-point grid was used for sampling the Brillouin zone. The antiferromagnetic phase was also investigated using the same parameters as used in the magnetic disordered phase to clearly see the effects of magnetic disorder, without considering the significant structural distortion the antiferromagnetic MnO54 undergoes.We not only implemented spin noncollinearity into the MSM approach, but also introduced a sample averaging scheme in the canonical ensemble, in which a new configuration contributed to the physical properties depending on the weight of each magnetic sample proportional to the Boltzmann factor. Then, any physical quantity in the paramagnetic phase, XPM, was evaluated by an ensemble average defined by
![[scr Z, script letter Z]](https://www.rsc.org/images/entities/char_e14b.gif) is a partition function defined by
is a partition function defined by 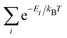 . Xi and Ei are the specific physical quantity and the energy of the ith microstate, respectively, and kB is the Boltzmann constant. In particular, the total energy, density of states (DOS) and local magnetic moment distributions were all computed by taking the ensemble average. We used seven different disordered structures for the ensemble average at T = 300 K. The probability of finding each microstate was in the range of 12 to 16% at room temperature, implying that there was no certain specific microstate dominant in the ensemble of paramagnetic MnO (see Fig. S1 in the ESI†).
. Xi and Ei are the specific physical quantity and the energy of the ith microstate, respectively, and kB is the Boltzmann constant. In particular, the total energy, density of states (DOS) and local magnetic moment distributions were all computed by taking the ensemble average. We used seven different disordered structures for the ensemble average at T = 300 K. The probability of finding each microstate was in the range of 12 to 16% at room temperature, implying that there was no certain specific microstate dominant in the ensemble of paramagnetic MnO (see Fig. S1 in the ESI†).
3 Results and discussion
3.1 Electronic structures of paramagnetic MnO
Fig. 2(a) shows the DOS of paramagnetic MnO determined using the NCMSM+U calculation. We chose the on-site Coulomb interaction to be U = 4 eV. This value enabled us to obtain results that agree well with the experimental measurements, which will be shown in the following subsections. The paramagnetic MnO DOS calculated by the NCMSM+U approach reveals features distinct from that of its antiferromagnetic counterpart shown in Fig. 2(b). It displays a wider bandwidth and a narrower band gap in the paramagnetic state compared to those in the antiferromagnetic state. Moreover, unlike the DOS of the antiferromagnetic phase, the DOS of the paramagnetic phase exhibits delocalized electronic characteristics in the valence band, as seen in Fig. 2(a). Such behavior gives rise to crucial consequences for electron correlations in the strongly-correlated MnO. It is noteworthy that, for the paramagnetic MnO, our numerical results are in good agreement with previous estimates from DFT+DMFT39 and the experimental XPS spectra.39 This result can be considered a step-up improvement in static DFT+U based calculations for strongly correlated systems. In usual DFT calculations, nonmagnetic states have been used to mimic paramagnetic states. We checked that the nonmagnetic MnO is metallic even in the presence of the Hubbard corrections (see Fig. S2 in the ESI†). This is consistent with the previous knowledge that DFT+U requires spin- or orbital-ordered states. Our NCMSM+U method provides an alternative way of achieving what the DFT+DMFT detects.3.2 Effects of electron correlations
We investigated the effects of strong electron correlations on the electronic structure of the paramagnetic MnO by adjusting the Hubbard parameter U from 0 to 7 eV. Fig. 3(a) displays the total and partial DOSs of the paramagnetic MnO for various U values. The majority-spin (minority-spin) bands shift down (up) with U. Here, the valence and conduction bands of MnO correspond one-to-one with the majority- and minority-spin bands, since the Mn ions have d5 high-spin configuration. In addition, the band shift is accompanied by a band gap increase. The band gap here is of the charge-transfer type. At a relatively high U, the system enters a charge transfer state with a minority-band shift from the Mott-Hubbard phase, as seen in Fig. 3(a). Thus, our results suggest that the paramagnetic MnO is a mixed type of Mott insulator with a band gap of 2.6 eV.We also investigated the electron correlation effect on the magnetic properties of paramagnetic MnO. We produced a canonical ensemble composed of individual microstates or paramagnetic configurations, which were generated through the self-consistent procedure performed using our NCMSM+U approach. The initial magnetic moments were chosen to be 4.58 μB, corresponding to the experimental value. Fig. 3(b) shows the distribution of the local magnetic moments calculated with different values of U. It is clearly shown that the choice of U values determines the distribution of the local magnetic moments in paramagnetic states. The mean value of each magnetic moment distribution obtained for a given U increases monotonically from 4.33 to 4.73 μB with U. This result is consistent with the previous knowledge that the on-site Coulomb repulsion enhances the spin- and orbital-polarization.55 On the other hand, the distribution width decreases with increasing U, that is, the local magnetic moments are widely (narrowly) distributed for small (large) values of U. From our NCMSM+U calculation, we determined an on-site Coulomb parameter of U = 4 eV, because this value yields a mean magnetic moment value of 4.59 μB, which is the closest value to the experimental value.
The interatomic superexchange couplings are directly related to the on-site Coulomb interactions. We extract the exchange coupling constants by mapping the energies of disordered states onto the Heisenberg Hamiltonian, obtained in the limit of U → ∞,
 | (2) |
3.3 Estimation of Néel temperature
To estimate the Néel temperature TN, which is the transition temperature from the antiferromagnetic (T < TN) to the paramagnetic state (T > TN), we evaluated the Helmholtz free energies FAFM and FPM of the antiferromagnetic and paramagnetic states in MnO as a function of temperature T. In general, the Helmholtz free energy F of a system, given by F = E − TS, can be determined by computing the internal energy E, and the entropy S at a given temperature T. The entropy S(T) of a magnetic system under the mean-field approximation can be expressed asS(T) = kB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln[M(T) + 1], ln[M(T) + 1], | (3) |
| FAFM(T) = EAFM, |
FPM(T) = EPM(T) − kBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) ln[M(T) + 1], ln[M(T) + 1], |
In addition, we estimated the crossover temperatures for several different values of U. The corresponding free energies at each U are presented in Fig. S3 of the ESI.† As shown in Fig. 4(b), the Néel temperature decreases from 250 K to 50 K with increasing U. This trend is closely related to the weakening of the exchange interactions. For comparison, we also estimated the Néel temperature at the PBE level. The result was more than twice that obtained with our NCMSM+U approach, which is consistent with the previous PBE-based result.44
3.4 Importance of spin noncollinearity in paramagnetic MnO
The introduction of spin noncollinearity is a major feature of our NCMSM+U approach compared to the conventional MSM. It is well known that, in most magnetic systems, the exchange interactions can be described by the bilinear exchange interactions. However, in some systems, the biquadratic exchange interactions cannot be ignored. The Heisenberg Hamiltonian that contains the biquadratic terms is generally expressed as | (4) |
To examine the effects of the spin noncollinearity, we compared our computational results of paramagnetic MnO obtained by the NCMSM+U approach to those by the MSM+U approach. We designed all the computational details identically except for the spin noncollinearity. Fig. 5 shows the DOS of the paramagnetic state (a) computed by the conventional MSM+U approach and that of the antiferromagnetic state (b). Even the conventional MSM+U approach described the delocalized valence band, which is a significant feature of paramagnetic MnO, as shown in Fig. 2(a). However, a remnant peak, which was not observed from NCMSM+U, appears at the top of the valence band in the result of MSM+U. Furthermore, we also estimated the Néel temperature based on the collinear calculations using the same method as described in Sec. 3.3. Here, the Néel temperature was estimated to be TN ≈ 189 K, which is 60% higher than the experimental Néel temperature. Note that the Néel temperature estimated from our noncollinear calculation is almost equal to the experimental value. These results indicate that the biquadratic exchange interaction is significant in MnO and, thus, spin noncollinearity should be taken into account for accurate prediction of the physical properties of paramagnetic MnO.
It is noteworthy that the antiferromagnetic state of MnO does not require spin noncollinearity even if the biquadratic exchange interactions are considered. MnO has type-II antiferromagnetic ordering at low temperature, in which the magnetic moments are all aligned in parallel. As a result, the spin-correlation functions of the collinear and noncollinear models are exactly the same: 〈Φα〉 represents a specific value depending on the coordination shell and 〈Ψα〉 is always 1. We confirmed that the DOS of antiferromagnetic MnO based on the collinear model shown in Fig. 5(b) is equivalent to that based on the noncollinear counterpart shown in Fig. 1(b).
4 Conclusions
We employed the noncollinear magnetic sampling method (NCMSM) with DFT+U (NCMSM+U) to calculate the paramagnetic Mott insulating state of MnO and investigated its room-temperature properties. The NCMSM+U approach accurately predicts the physical properties of paramagnetic Mott insulator MnO at reasonable computational cost. Specifically, we estimated the electronic profile, distribution of local magnetic moments, superexchange coupling constants, and Néel temperature. Furthermore, our work demonstrated that the inclusion of spin noncollinearity plays a crucial role in accurate description of paramagnetic Mott insulators where superexchange interactions exist. It significantly affects the detailed electronic profile and precise energetics of paramagnetic MnO. The NCMSM+U approach is of value in terms of scalability to other supercell calculations as compared to KKR-CPA or DFT+DMFT. We, therefore, expect our results to help in providing a basis for the rational design of energy materials using paramagnetic Mott insulators. It should be noted that the NCMSM+U approach cannot be used for paramagnetic metals with fast dynamic charge correlations.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from the Korean government through National Research Foundation (2017R1A2B3011629, 2019R1A2C1005417, 2017M3D1A1040688), and from the Ministry of Trade, Industry & Energy (MOTIE) of Korea (Project No. 10080625). Some portion of our computational work was done using the resources of the KISTI Supercomputing Center (KSC-2018-C2-0033 and KSC-2018-CHA005).References
- Y. Gorlin and T. F. Jaramillo, J. Am. Chem. Soc., 2010, 132, 13612–13614 CrossRef CAS PubMed.
- I. Zaharieva, P. Chernev, M. Risch, K. Klingan, M. Kohlhoff, A. Fischer and H. Dau, Energy Environ. Sci., 2012, 5, 7081–7089 RSC.
- A. Bergmann, I. Zaharieva, H. Dau and P. Strasser, Energy Environ. Sci., 2013, 6, 2745–2755 RSC.
- M. Wiechen, M. M. Najafpour, S. I. Allakhverdiev and L. Spiccia, Energy Environ. Sci., 2014, 7, 2203–2212 RSC.
- M. Huynh, C. Shi, S. J. L. Billinge and D. G. Nocera, J. Am. Chem. Soc., 2015, 137, 14887–14904 CrossRef CAS PubMed.
- A. Indra, P. W. Menezes, I. Zaharieva, E. Baktash, J. Pfrommer, M. Schwarze, H. Dau and M. Driess, Angew. Chem., Int. Ed., 2013, 52, 13206–13210 CrossRef CAS PubMed.
- K. Jin, A. Chu, J. Park, D. Jeong, S. E. Jerng, U. Sim, H. Y. Jeong, C. W. Lee, Y. S. Park, K. D. Yang, G. K. Pradhan, D. Kim, N. E. Sung, S. H. Kim and K. T. Nam, Sci. Rep., 2015, 5, 10279 CrossRef PubMed.
- K. Jin, H. Seo, T. Hayashi, M. Balamurugan, D. Jeong, Y. K. Go, J. S. Hong, K. H. Cho, H. Kakizaki, N. Bonnet-Mercier, M. G. Kim, S. H. Kim, R. Nakamura and K. T. Nam, J. Am. Chem. Soc., 2017, 139, 2277–2285 CrossRef CAS PubMed.
- A. L. M. Reddy, M. M. Shaijumon, S. R. Gowda and P. M. Ajayan, Nano Lett., 2009, 9, 1002–1006 CrossRef CAS PubMed.
- L. Li, A. R. O. Raji and J. M. Tour, Adv. Mater., 2013, 25, 6298–6302 CrossRef CAS PubMed.
- X. Gu, L. Chen, Z. Ju, H. Xu, J. Yang and Y. Qian, Adv. Funct. Mater., 2013, 23, 4049–4056 CrossRef CAS.
- H. Wang, L. F. Cui, Y. Yang, H. Sanchez Casalongue, J. T. Robinson, Y. Liang, Y. Cui and H. Dai, J. Am. Chem. Soc., 2010, 132, 13978–13980 CrossRef CAS PubMed.
- J. Gao, M. A. Lowe and H. D. Abruna, Chem. Mater., 2011, 23, 3223–3227 CrossRef CAS.
- M. H. Oh, T. Yu, S. H. Yu, B. Lim, K. T. Ko, M. G. Willinger, D. H. Seo, B. H. Kim, M. G. Cho, J. H. Park, K. Kang, Y. E. Sung, N. Pinna and T. Hyeon, Science, 2013, 340, 964–968 CrossRef CAS PubMed.
- X. Li, D. Li, L. Qiao, X. Wang, X. Sun, P. Wang and D. He, J. Mater. Chem., 2012, 22, 9189–9194 RSC.
- B. Liu, X. Hu, H. Xu, W. Luo, Y. Sun and Y. Huang, Sci. Rep., 2014, 4, 4229 CrossRef PubMed.
- X. Zhao, Y. Du, L. Jin, Y. Yang, S. Wu, W. Li, Y. Yu, Y. Zhu and Q. Zhang, Sci. Rep., 2015, 5, 14146 CrossRef CAS PubMed.
- S. Chen, J. Zhu, X. Wu, Q. Han and X. Wang, ACS Nano, 2010, 4, 2822–2830 CrossRef CAS PubMed.
- Y. He, W. Chen, X. Li, Z. Zhang, J. Fu, C. Zhao and E. Xie, ACS Nano, 2012, 7, 174–182 CrossRef PubMed.
- G. Yu, L. Hu, N. Liu, H. Wang, M. Vosgueritchian, Y. Yang, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 4438–4442 CrossRef CAS PubMed.
- L. Bao, J. Zang and X. Li, Nano Lett., 2011, 11, 1215–1220 CrossRef CAS PubMed.
- P. Xu, B. Wei, Z. Cao, J. Zheng, K. Gong, F. Li, J. Yu, Q. Li, W. Lu and J.-H. Byun, ACS Nano, 2015, 9, 6088–6096 CrossRef CAS PubMed.
- A. I. Liechtenstein, V. I. Anisimov and J. Zaanen, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 52, 5467–5470 CrossRef.
- S. L. Dudarev, G. A. Botton, S. Y. Savrasov, C. J. Humphreys and A. P. Sutton, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 1505 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 1372–1377 CrossRef CAS.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- S. Massidda, A. Continenza, M. Posternak and A. Baldereschi, Phys. Rev. Lett., 1995, 74, 2323–2326 CrossRef CAS PubMed.
- A. Filippetti and N. A. Spaldin, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 125109 CrossRef.
- C. Franchini, R. Podloucky, J. Paier, M. Marsman and G. Kresse, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 195128 CrossRef.
- V. Bayer, C. Franchini and R. Podloucky, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 035404 CrossRef.
- D. A. Tompsett, D. S. Middlemiss and M. S. Islam, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 205126 CrossRef.
- C. Ling and F. Mizuno, Chem. Mater., 2012, 24, 3943–3951 CrossRef CAS.
- D. A. Tompsett, S. C. Parker, P. G. Bruce and M. S. Islam, Chem. Mater., 2013, 25, 536–541 CrossRef CAS.
- M. Pavone, A. B. Munoz-Garcia, A. M. Ritzmann and E. A. Carter, J. Phys. Chem. C, 2014, 118, 13346–13356 CrossRef CAS.
- C. Ling, R. Zhang, T. S. Arthur and F. Mizuno, Chem. Mater., 2015, 27, 5799–5807 CrossRef CAS.
- Z. Xu, J. Rossmeisl and J. R. Kitchin, J. Phys. Chem. C, 2015, 119, 4827–4833 CrossRef CAS.
- S. Y. Savrasov and G. Kotliar, Phys. Rev. Lett., 2003, 90, 056401 CrossRef CAS PubMed.
- X. Ren, I. Leonov, G. Keller, M. Kollar, I. Nekrasov and D. Vollhardt, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 195114 CrossRef.
- J. Kuneš, A. V. Lukoyanov, V. I. Anisimov, R. T. Scalettar and W. E. Pickett, Nat. Mater., 2008, 7, 198–202 CrossRef PubMed.
- J. Shim, K. Haule and G. Kotliar, Science, 2007, 318, 1615–1617 CrossRef CAS PubMed.
- K. Haule, C. H. Yee and K. Kim, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 195107 CrossRef.
- M. Capone, M. Fabrizio, C. Castellani and E. Tosatti, Phys. Rev. Lett., 2004, 93, 047001 CrossRef PubMed.
- A. I. Lichtenstein and M. I. Katsnelson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 9283–9286 CrossRef.
- B. Alling, T. Marten and I. A. Abrikosov, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 184430 CrossRef.
- P. Steneteg, B. Alling and I. A. Abrikosov, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 144404 CrossRef.
- L. Zhou, F. Körmann, D. Holec, M. Bartosik, B. Grabowski, J. Neugebauer and P. H. Mayrhofer, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 184102 CrossRef.
- E. Mozafari, B. Alling, P. Steneteg and I. A. Abrikosov, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 094101 CrossRef.
- I. Hughes, M. Däne, A. Ernst, W. Hergert, M. Lüders, J. Staunton, Z. Szotek and W. Temmerman, New J. Phys., 2008, 10, 063010 CrossRef.
- B. Frandsen, M. Brunelli, K. Page, Y. Uemura, J. Staunton and S. Billinge, Phys. Rev. Lett., 2016, 116, 197204 CrossRef PubMed.
- A. K. Cheetham and D. A. O. Hope, Phys. Rev. B: Condens. Matter Mater. Phys., 1983, 27, 6964–6967 CrossRef CAS.
- G. Kresse and J. Furthmuller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. L. Dudarev, P. Liu, D. A. Andersson, C. R. Stanek, T. Ozaki and C. Franchini, 2018, arXiv:1811.06864.
- H. Shaked, J. Faber Jr. and R. Hitterman, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 38, 11901–11903 CrossRef CAS.
- V. I. Anisimov, J. Zaanen and O. K. Andersen, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 44, 943–954 CrossRef CAS.
- M. E. Lines and E. D. Jones, Phys. Rev., 1965, 139, 1313–1327 CrossRef CAS.
- M. Kohgi, Y. Ishikawa and Y. Endoh, Solid State Commun., 1972, 11, 391–394 CrossRef CAS.
- D. Bloch and R. Maury, Phys. Rev. B: Condens. Matter Mater. Phys., 1973, 7, 4883–4888 CrossRef CAS.
- B. Boucher, R. Buhl and M. Perrin, J. Phys. Chem. Solids, 1971, 32, 2429–2437 CrossRef CAS.
- G. Pepy, J. Phys. Chem. Solids, 1974, 35, 433–444 CrossRef CAS.
- C. S. Yoo, B. Maddox, J. H. P. Kiepeis, V. Iota, W. Evans, A. McMahan, M. Y. Hu, P. Chow, M. Somayazulu, D. Hausermann, R. T. Scalettar and W. E. Pickett, Phys. Rev. Lett., 2005, 94, 115502 CrossRef CAS PubMed.
- P. W. Anderson, Phys. Rev., 1950, 79, 350–356 CrossRef.
- N. L. Huang and R. Orbach, Phys. Rev. Lett., 1964, 12, 275 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cp00280d |
| ‡ Present address: Korea Institute for Advanced Study (KIAS), Seoul, 02455, Korea. |
| § Present address: Department of Physics, University of Arizona, Tucson, AZ, 85721, USA. |
| This journal is © the Owner Societies 2019 |





