Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Macroscopic defects upon decomposition of CO2 clathrate hydrate crystals†
Stefan
Arzbacher
 *ab,
Nima
Rahmatian
*ab,
Nima
Rahmatian
 a,
Alexander
Ostermann
a,
Alexander
Ostermann
 c,
Bernhard
Massani
d,
Thomas
Loerting
c,
Bernhard
Massani
d,
Thomas
Loerting
 b and
Jörg
Petrasch
a
b and
Jörg
Petrasch
a
aIllwerke vkw Endowed Professorship for Energy Efficiency, Research Center Energy, Vorarlberg University of Applied Sciences, Hochschulstraße 1, Dornbirn 6850, Austria. E-mail: stefan.arzbacher@fhv.at; Tel: +43 5572 792 3803
bInstitute of Physical Chemistry, University of Innsbruck, Innrain 52c, Innsbruck 6020, Austria
cDepartment of Mathematics, University of Innsbruck, Technikerstraße 13, Innsbruck 6020, Austria
dInstitute for Condensed Matter and Complex Systems, University of Edinburgh, EH9 3JZ, Edinburgh, UK
First published on 26th April 2019
Abstract
Micrometer- and submicrometer-sized pores and macroscopic defects like cracks and tubular channels can be found in a variety of clathrate hydrates (hydrates for short) during formation and decomposition. Their origin, their evolution in time, and their effect on hydrate decomposition kinetics are unclear. We used time-lapse micro computed tomography (μCT) in combination with temperature control and pressure monitoring to study the formation and evolution of pores and macroscopic defects in decomposing CO2 hydrates at subzero (Celsius) temperature. Our results suggest that the decomposition of hydrates is always accompanied by the formation of pores and an increase of the apparent hydrate volume by more than 3%. Hydrate decomposition often starts with the formation of cracks inside the hydrate and not necessarily at the free surface of the hydrate, which frequently remains intact for a long period and seems to be self-preserved in some regions. Decomposition spreads out from these cracks in a uniform fashion yielding a variety of macroscopic features. In some cases, the propagating decomposition front seems to get blocked by planar barriers inside the hydrate yielding regions with high resistance against decomposition. This, together with a generally heterogeneous distribution of decomposition resistant regions, challenges the shrinking core model of hydrate decomposition as well as the popular ice-rind theory used to explain the effect of self-preservation.
1 Introduction
Clathrate hydrates, or hydrates for short, are crystalline, ice-like solids comprising water and small-sized guest molecules, most prominently light hydrocarbons or carbon dioxide.1–3 Their structure is determined by a hydrogen-bonded network of water molecules that form polyhedra (or cavities) which are stabilized by the guest molecules. In the case of CO2 as the guest molecule, six large polyhedra (truncated hexagonal trapezohedra) and two small polyhedra (pentagonal dodecahedra) form a unit cell of the cubic structure termed sI.4Hydrates are generally non-stoichiometric compounds, meaning that not all polyhedra have to be occupied by a guest molecule. However, in the ideal stoichiometric case all cavities of a CO2 hydrate are occupied by exactly one guest molecule. That gives a ratio of 8 guest molecules per 46 water molecules or CO2·5.75H2O. Combined with a theoretical stoichiometric density1 of 1.13 g cm−3 this yields 7.7 kilomoles of CO2 per cubic meter of hydrate. Thus, at standard temperature and pressure (STP) conditions 174 volumes of gaseous CO2 are stored in one volume of CO2 hydrate. For comparison, dry ice accumulates approximately 796 volumes of gas (STP) per volume of solid.
This high density of CO2 combined with stability at moderate pressures and medium temperatures (e.g. 1.2 MPa at 273 K)1 makes CO2 hydrate an interesting material for several industrial applications. The food industry has tried to produce carbonated ice (i.e., CO2 hydrate) as a means for home production of soft drinks since the 1950s.5 Although a real product is still hard to find, their efforts have continued also in recent years.6 Hatakeyama et al.7 showed that CO2 hydrate can be effectively used for fire fighting. The large heat of hydrate dissociation (∼60 kJ mol−1)8 cools down the flame while the released non-flammable gas reduces the supply of oxygen. Another application making use of the large enthalpy of hydrate dissociation is in refrigeration systems where a CO2 hydrate slurry is the two-phase refrigerant with minimal impact on the environment.9,10 The largest field of possible future application is the sequestration of CO2 from pre- and post-combustion gas in hydrate form combined with subsequent long term storage in deep geological formations or the deep ocean. Although this carbon capture technology is currently too energy intensive for industrial use, this may change due to the development of hybrid technologies or new additives, which can significantly reduce the hydrate formation pressures.11–15
Besides artificially formed CO2 hydrate, there are also natural occurrences. On Earth, they have been found at the sea floor in hydrothermal fields where CO2 rich gas bubbles are emitted from volcanic sources.16 Though direct evidence is still missing, extraterrestrial occurrences of CO2 hydrate on icy moons (e.g. Europa,17 and the satellites of Saturn18,19), comets,20,21 and on Mars22 are assumed mainly based on the existence of both CO2 and water together with temperature conditions which prevent the hydrate from dissociation.
Although phase equilibrium data are well established over a wide range of temperatures,1 the processes involved in nucleation, formation, and decomposition of hydrates remain unclear even with a large amount of experimental work using a variety of techniques.23 For instance, hydrate gas release and dissociation rates are determined using both macroscopic pVT-work24–30 and time-resolved diffraction measurements.31–36 In the case of neutron diffraction in situ experiments were done even at elevated pressures,37,38 revealing phase fractions of hydrate and ice and identifying metastable intermediate phases like cubic ice (ice Ic). Micro-computed X-ray tomography (μCT) can be used to capture the three-dimensional structure of hydrate samples.39,40 Phase-shift information is added in diffraction-enhanced X-ray imaging (DEI) to overcome the common problem of poor contrast between hydrate and ice/water in conventional attenuation-based μCT.41,42 Optical microscopy and visual observations of hydrates are used to investigate changes of the surface texture during decomposition.27,28,43 More detailed information on the surface microstructure on the micrometer and submicrometer scale is collected using cryo scanning electron microscopy (SEM).25,26,29,37,38,43 The local interaction between guest and host molecules is usually examined using Raman or infrared spectrometry.44,45
Focusing on decomposition, the difficulty until today is mainly finding a meaningful relation between dissociation kinetics and thermodynamic state variables. Clathrate hydrates of guests which are gaseous at STP conditions decompose into gas and ice (T < 273.2 K) or gas and water (T > 273.2 K) when they are placed outside their stability region by either superheating (T > Teq.) or under-pressurizing (p < peq.). The assumption that the stronger the driving force (i.e., the chemical potential difference associated with superheating or under-pressurizing), the higher the dissociation rate does not hold in general. In fact, some hydrates (CH4, CO2, Kr, Ar, O2, N2 and others33) show very small rates of dissociation outside the stability field, but both at lower and higher temperatures, rates are significantly larger. Davidson et al.46 first observed this phenomenon in calorimetry experiments with natural gas and methane hydrates at 240 < T < 273 K while Handa et al.47 reported the same for Kr hydrates at 225 < T < 273 K shortly after. They argued that the ice produced in the initial period of decomposition protects the remaining hydrate from further dissociation by forming an impenetrable coating. This effect was later termed “self-preservation” by Yakushev and Istomin48 and since then has been observed many times by numerous researchers.24,25,29–31,38,49–51
The abrupt change in dissociation rates at the onset temperature of the self-preservation effect, as well as two distinct minima within the self-preservation regime,24,52,53 made the development of a working hypothesis for hydrate decomposition difficult. Today it seems established that ice as a hydrate decomposition product is a crucial factor in hydrate decomposition and self-preservation in particular. This is also the foundation of two popular hypotheses: (1) Kuhs et al.37 found that the ice formed during decomposition is generally defective and stacking-faulty up to T ≈ 240 K. They concluded that below 240 K the ice cannot act as an efficient diffusion barrier, while above 240 K annealing turns it into a rather dense and impermeable shield of hexagonal ice (ice Ih). Later, Falenty and Kuhs38 suggested that the annealing of defective ice combined with a variety of microstructures found on freshly formed ice might lead to various decomposition paths including one with a very pronounced effect of preservation. (2) In several studies Melnikov et al.27,28,54 showed that liquid water formation during hydrate dissociation is possible in the temperature range from 253 K to 273 K. Furthermore they showed that due to a high energy barrier, the solid–solid transformation of hydrate to ice is unlikely in a metastable region bounded by the three phase equilibrium curves of ice–hydrate–gas and water–hydrate–gas extended to temperatures below 273.2 K. Since supercooled liquid water can exist in the temperature range from 233 K to 273 K at 0.1 MPa, they proposed a relation between supercooled liquid water formation and the effect of self-preservation and suggested that freezing of a layer of supercooled water generates the gas-impermeable ice shell.
Additionally, pores and macroscopic defects like cracks and tubes on the micrometer and submicrometer scale have been found in several studies on natural and synthetic hydrates during both formation and decomposition.37,42,55–61 Although pores seem to be characteristic of hydrates, to our knowledge, no serious attempts have been made so far to study the influence of pores on the decomposition behavior. The same is true for cracks, which seem to act as nucleation sites for hydrate decomposition.42,60,61
We use time-resolved attenuation-based μCT to study the formation and evolution of pores and macroscopic defects in decomposing CO2 hydrates at different conditions inside and outside the self-preservation regime (from 240 K to 273 K). The samples were produced using vapor co-deposition of water and CO2.62 The three-dimensional images are combined with pT-data collected from our in situ setup described in ref. 63. The pressure data are used to correlate the volume of decomposed hydrate with the amount of gas released. Although submicrometer-sized pores are too small to be detected directly with the resolution used (a voxel edge length of 6 μm), we can still infer their existence. This is because the formation of pores in decomposing hydrate is accompanied by a significant change in apparent density when compared to hexagonal ice. The unique setup used herein offers a combination of non-destructive long-term observation, accurate mass balance due to a closed system, and highly controlled sample history. It is applied to understand the role of pores, cracks and other macroscopic defects in the decomposition process of hydrates and self-preservation in particular.
2 Experimental
Sample preparation, the tomographic setup, and the experimental procedures used are briefly described in this section. Details on μCT settings, temperature and mass calibration, leakage, the non-uniform temperature field, and segmentation error are discussed in the ESI† provided with this document.2.1 Sample preparation
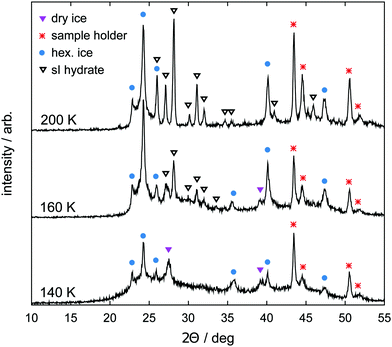
Samples are prepared using a vapor co-deposition technique as described in ref. 62, 64 and 65. Water vapor is deposited as amorphous solid water (ASW) together with CO2 on a copper sample holder cooled to 77 K in a vacuum system. The co-deposition is done for 12 h at partial pressures p(H2O) = 0.20 mbar and p(CO2) = 0.02 mbar. The base pressure of the high vacuum chamber is 10−4 mbar. Subsequent warming of the deposit to 140 K in the vacuum causes the ASW to sinter and to efficiently capture CO2 in the pore space of the ASW. Additionally, it causes the desorption of CO2 adsorbed on external surfaces. This hydrate precursor material is then cooled back to 77 K, recovered after venting with dry nitrogen and stored in liquid nitrogen until needed in the experiments.Samples of the hydrate precursor material are characterized by powder X-ray diffraction (PXRD). To this end, the samples are powdered under liquid nitrogen and transferred to the sample holder using a cryospoon. The sample holder is made from nickel-plated copper, precooled to about 80 K and allows for the powder to remain in a horizontal position for the whole measurement. The sample holder is part of the low temperature chamber TTK450 from Anton Paar GmbH. PXRD measurements are done on a Siemens D5000 instrument equipped with a Cu Kα1 X-ray source (wavelength 1.5406 Å) operated at 40 kV and 40 mA in θ/2θ scanning mode in the 2θ range from 10° to 55° (step width 0.02°). Installation of a Göbel mirror for parallel beam optics allows for measurement of undistorted Bragg peaks even for small amounts of powdered sample. For measurement, the chamber is evacuated to below 1 mbar, where the valve to the pump system is closed for the measurement itself (“static vacuum”). The temperature is regulated by controlling resistive heaters at the sample holder as well as the liquid nitrogen flow through the TTK450 chamber using a needle valve and a temperature controller (LakeShore, model CI330). As shown in Fig. 1, the precursor material transforms to CO2 hydrate and hexagonal ice during the loading procedure as the temperature reaches 200 K.
2.2 Tomographic setup
A lab-scale GE nanotom-m μCT system is used to perform full rotation scans of the samples. All scans are done using a tube voltage of 70 kV and a geometrical magnification factor of 16.7 resulting in a voxel edge length of 6 μm.The manipulator of the μCT is equipped with a slightly improved version of the custom-built cryo stage designed for our recent study.63 The stage (cf.Fig. 2) is cooled by a stack of thermoelectric Peltier elements (QC-31-1.0-3.9MS and QC-17-1.4-3.7MS, Quick-Ohm Germany) as well as a flow of cold gaseous nitrogen (N2(g)). N2(g) is produced from liquid nitrogen within the closed X-ray cabin using two independent custom-built evaporators ensuring a controlled flow of N2(g).
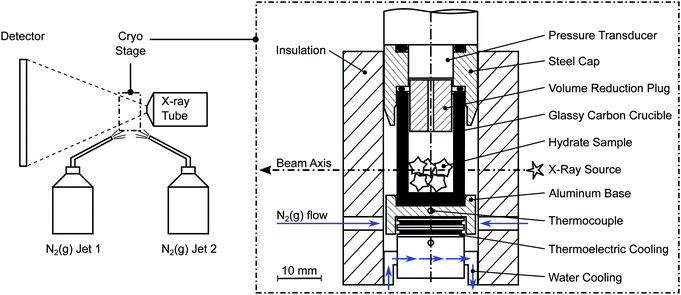 | ||
| Fig. 2 Schematic of the tomographic setup (left) together with a cross sectional view of the cryo stage (right). | ||
The pressure vessel of the cryo stage consists of a glassy carbon crucible (SIGRADUR G, HTW Germany) and a steel cap fitted together using a two-component epoxy adhesive. It is referred to as the cell for the rest of this manuscript. Glassy carbon is chosen as the cell wall material since it is highly transparent to X-rays, impermeable to gases and liquids, extremely resistant to thermal shocks, and strong enough to withstand high pressures in a wide range of temperatures. An aluminum base, in good thermal contact with the glassy carbon crucible, is cooled using both N2(g) flow and thermoelectric cooling. The latter is primarily used to avoid temperature fluctuation. The sample temperature is measured with an accuracy of 1 K by a calibrated thermocouple (K-type, d = 1 mm) positioned in the center of the aluminum base. A pressure transducer (PXM459, OMEGA Germany) with an accuracy of 6 mbar is connected to the steel cap of the cell. Due to the small cell volume (1.90 mL) even minor amounts of gas release can be registered with an accuracy of 1 μg. The experiment is monitored, controlled, and logged at a frequency of 1 Hz using a commercial data acquisition system (NI cRIO-9022, National Instruments USA). The setup has been tested to 10 MPa and can be used to cool samples down to 180 K.
The temperature gradient in the setup and the sample, a unique feature of our setup, is a direct consequence of space limitations in the μCT cabin and our requirements of (1) the highest possible magnification in the μCT, (2) maximal pressure resolution, and (3) accessibility of a wide range of temperatures and pressures.
2.3 Image processing
Radiograms collected during the μCT measurements are used by GE's phoenix datos|x reconstruction software (GE Sensing & Inspection Technologies, Germany) to compute the three-dimensional raster image of the sample with a voxel edge length of 6 μm. The image data is filtered with a three dimensional median filter with a kernel size of 7 voxels in order to enhance the signal to noise ratio. The random walk segmentation algorithm of Grady66 is applied subsequently to segment the image data into gas, hydrate, and decomposed hydrate/ice phases. In this segmentation algorithm each voxel of the image data is classified based on its gray scale value and that of the neighboring voxels. It is particularly useful in preserving the connectedness of regions even in the presence of unwanted image noise. The process is shown in Fig. S6 (ESI†) together with further details about the individual steps and a brief discussion on segmentation error.2.4 Procedure
Three different pathways, namely a temperature stepping one (cf.Fig. 3) and two isothermal (cf.Fig. 4) ones, are used in our experiments. The idea consists of the following loop: stable state → structure capture via μCT → destabilization → decomposition and pressure rise → stabilization at the vapor–ice–hydrate (V–I–H) equilibrium pressure → stable state. In the case of the temperature stepping pathway the hydrate is destabilized by overheating with respect to the stability temperatures. In experiments following the isothermal pathway, the destabilization is triggered by rapid depressurization from the stability pressures.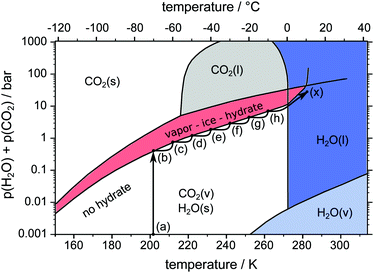 | ||
| Fig. 3 pT-pathway of the temperature stepping procedure. After loading the sample at point (a) the equilibrium pressure is achieved at point (b). Starting from there the temperature is raised in steps of 10 K (b–h) leading to new equilibrium pressures. Eventually the sample is heated across the melting point (h–x). The phase diagram of the two-component system H2O–CO2 is based on data and theoretical predictions from ref. 1. | ||
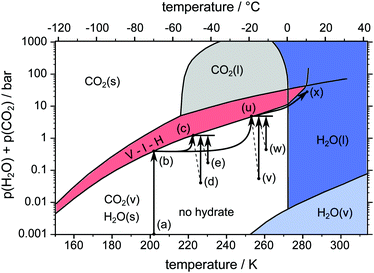 | ||
| Fig. 4 pT-pathway of the isothermal procedures. After loading the sample at point (a) the equilibrium pressure is achieved at point (b). Subsequently the temperature is rapidly raised to the isothermal set point temperature accompanied by new equilibrium pressures (point (c) or (u)). Rapid depressurization to points (d), (e), (v) and (w) results in further gas release until equilibrium pressures are restored. Eventually, the samples are heated across the melting point towards point (x). The phase diagram of the two-component system H2O–CO2 is based on data and theoretical predictions from ref. 1. Note that pressures at points (d), (e), (v) and (w) are not to scale. An explanation for differing pressures after rapid depressurization is given in the text. Points (d), (e), (v), and (w) are shifted in temperature for reasons of readability only. | ||
In all pathways, loading is done by transferring the sample from the liquid nitrogen dewar directly into the precooled (203 K) cell. The cell is tightly closed immediately after the transfer. This is accompanied by a very sharp increase of pressure due to the evaporation of liquid nitrogen. In order to release the evaporated nitrogen the cell is opened once or twice after loading. Within less than one minute no further evaporation is observed and the experiment is started with the cell containing mainly N2(g). After loading, the sample is outside the stability region at point (a) so the decomposition starts with a release of gas, resulting in an increase of pressure in the closed cell. The pressure continues to rise until it reaches the V–I–H equilibrium pressure of 0.43 bar1 at point (b). There, the first μCT scan is done 15 min after the pressure stabilizes.
In the temperature stepping experiment (cf.Fig. 3) the temperature is repeatedly raised by 10 K at a rate of 2 K min−1 following the path (b–h). Every step in temperature causes further decomposition, release of gas, and pressure increase. At every step, a μCT scan is done once the new equilibrium pressure is reached after some time. Eventually, the sample is brought to full decomposition by heating (1 K min−1) across the melting point of ice.
Two different isothermal experiments are depicted as pathways (a-b-c-d-c-e-c-x) and (a-b-u-v-u-w-u-x) in Fig. 4. After an initial scan at point (b) the temperature is rapidly raised by 10 K min−1 to either 228 K at point (c) or 253 K at point (u). Again, the new equilibrium pressure is reached and a μCT scan is started. After the scan is completed, the cell is rapidly depressurized to point (d) or (v) at constant temperature by opening and closing. This destabilizes the hydrate and decomposition continues. When point (c) or (u) is attained once more, the current state of the hydrate structure is captured with another scan. The rapid depressurization procedure is repeated several times provided that the amount of remaining hydrate is enough to restore point (c) or (u) after one more depressurization. Note that the term “isothermal” here refers to parts (c-d-c-e-c) and (u-v-u-w-u) of the pathway, where the cell is kept at constant temperature for a long time. The isentropic cooling of the cell gas due to rapid depressurization is neglected in this description. Similar to temperature stepping, the isothermal experiments are terminated by heating the sample across the melting point of ice. The sum of partial pressures of CO2 and H2O immediately after rapid depressurization (points (d), (e), (v), and (w)) is below 1 atm (see Fig. 4). The difference to 1 atm refers to the partial pressure of the undetermined amount of N2(g) gas in the system. With every opening event this difference decreases due to escaping N2(g).
In both temperature stepping and isothermal experiments the total amount of water in the system is obtained based on the reconstructed volume from the last scan at point (x).
In all procedures, N2(g) as an additional component in the gas composition allows the investigation of CO2 hydrates at sub-atmospheric pressures without the use of a vacuum pump, which would have made the loading procedure severely more complicated. Besides, the self-preservation phenomenon in CO2 hydrate occurs at a variety of atmospheric conditions, including mixtures of CO2 and N2.36 Moreover, the formation pressure of N2 hydrate (e.g. 16 MPa at 273 K)1 is considerably larger than the maximal N2(g) partial pressure in our experiments (0.1 MPa). Additionally, the statistical thermodynamic modeling predictions of CSMGem1 point towards a neglectable amount of N2 in the sI hydrate phase at the conditions investigated. Therefore, we assume that no N2 is taken up in the CO2 hydrates of our experiments. Thus, the amount of nitrogen in the cell gas remains constant over the course of the experiment, provided that the cell is not opened. The absolute pressure and CO2 concentration, reflected as the CO2 partial pressure, influence the decomposition at the hydrate–gas interface. Here, it is supposed that pressure effects at the hydrate–gas interface are subordinate to concentration effects. As a consequence, we assume that the initial amount of nitrogen gas shifts the vapor–ice–hydrate (V–I–H) equilibrium pressures of the two component system CO2–H2O by a constant pressure offset.
2.5 Determination of the mass, composition, and temperature
The combination of accurate volume and pressure data enables us to determine the sample mass and sample composition. The mass of water m(H2O) in the sample is determined from the volume of liquid water VLiquid measured at 283 K at the end of the experiment and a liquid water density of 0.9997 g cm−3. The gas mass mCO2(t) released from the hydrate from the beginning of the experiment until time t is derived from the pressure signal using a fitted model equation for the mass–pressure relation (cf. ESI†). Gas leakage and dissolution of CO2 in water are neglected in the computation of mCO2. While the accuracy of m(H2O) is 1%, larger uncertainties are associated with the computation of mCO2 due to the unknown amount of N2(g) in the cell. To account for that, we use the subscripts min and max to refer to an initial N2(g) partial pressure of 1 atm and 0 atm, respectively. For instance, mmin,CO2 and mmax,CO2 denote the lower and upper bounds of mCO2 depending on the initial amount of N2(g) in the cell. Both mmin,CO2 and mmax,CO2 are accurate to within 1.5%. The largest pressure value observed immediately after the full decomposition of the hydrate at time t* (i.e., when all CO2 is in the gas phase) yields mmin(CO2) := mmin,CO2(t*) and mmax(CO2) := mmax,CO2(t*), the lower and upper bounds of the total mass of CO2 in the sample. Since the maximal pressure is usually found at temperatures slightly below 273 K the effect of dissolved CO2 on mmin(CO2) and mmax(CO2) is neglected. The knowledge of the water and guest gas mass allows the determination of the sample composition, i.e., the determination of n in CO2·nH2O. However, in cases of n > 5.75, this number cannot be used to differentiate between excess water in ice form and that in empty hydrate cages. From the powder X-ray diffraction (PXRD) patterns of Fig. 1 it is clear though that a small amount of ice is present in our samples. While the cell pressure and the sample volume can be measured directly, direct determination of the sample temperature is possible only in the lowest region of the sample (temperature of Tlow, cf. ESI†). Further above the bottom of the cell, temperatures are higher due to the temperature gradient along the cell axis (cf. ESI†). The temperature gradient reduces linearly from about 2.5 K mm−1 at Tlow = 203 K to approximately 0.5 K mm−1 at Tlow = 273 K. The highest temperature that the sample is exposed to is denoted as Thigh. Since in our setup Thigh cannot be determined directly, we use the settled experimental pressure to obtain a range Thigh,min < Thigh < Thigh,max of possible values (cf. ESI†). For simplicity all temperatures in the text refer to Tlow if not specified differently.3 Results and discussion
For clarity reasons, the results are presented in four subsections. First, a correlation between hydrate gas release and mesostructural change is established. Second, mesostructural changes between isothermal experiments at 228 K and 253 K are compared. Third, the evolution of macroscopic defects and pores is tracked. Fourth, close-up views of the initial phase of decomposition expressed by gas release rates are presented. Important key data of all experiments are summarized in Table 1.| Experiment | m(H2O) (mg) | m min(CO2) (mg) | m max(CO2) (mg) | CO2·nH2O (−) | V Sample,initial (mm3) | V Sample,end (mm3) | V Liquid (mm3) |
|---|---|---|---|---|---|---|---|
| Temp. stepping | 90.0 | 23.4 | 27.3 | 8.0 < n < 9.4 | 120.1 | 127.1 | 90.0 |
| Isothermal 228 K | 155.7 | 47.2 | — | n < 8.0 | 205.9 | 213.2 | 155.7 |
| Isothermal 253 K | 134.6 | 45.9 | — | n < 7.2 | 209.8 | 216.2 | 134.6 |
3.1 Temperature stepping
Two ice particles are added to a hydrate sample in a temperature stepping experiment in order to serve as a well determined reference for the ice phase. The mass of the ice particles is 19 mg and the total amount of water in the cell is 109 mg. Fig. 5 shows Tlow, the gauge pressure, and the released CO2 mass mmin,CO2 over the course of the experiment. μCT scans done at eight different temperatures are labeled with Roman numerals and tagged as blue dots on the temperature signal. The dotted line shows the theoretical value of the V–I–H equilibrium pressure corresponding to Tlow. Differences between measured and equilibrium pressures are caused by the non-uniform temperature field (cf. ESI†) and the initial amount of N2(g) introduced during the loading procedure.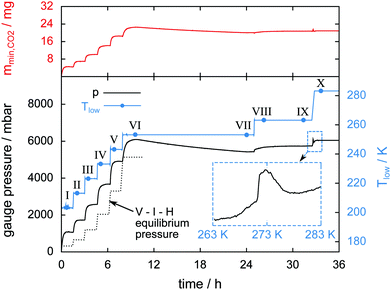 | ||
| Fig. 5 Gauge pressure, temperature Tlow, and mmin,CO2 (mass of the released CO2) as a function of time. Blue dots denote the start of a μCT scan labeled with Roman numerals. The inset shows the gauge pressure as a function of temperature during melting of the hydrate at a heating rate of 1 K min−1. The dotted line shows the theoretical V–I–H equilibrium pressure at the corresponding temperature as predicted by the software CSMGem.1 | ||
During the initial period of the experiment every increase in the sample temperature results in a period of significant gas release until the pressure settles at a new V–I–H equilibrium. This is observed until scan VI is done at 253 K after 10 h experiment time. In the period between 10 h and 24 h experiment time, the full halt of gas release leads to a small decrease of pressure caused by leakage. Remarkably, after scan VIII at 263 K, the pressure remains constant for more than 6 h. This implies that the leak of gas is now compensated by the gas release from the hydrate. The sample melting is shown in the inset of Fig. 5. Here, the gauge pressure is depicted as a function of temperature during heating at a rate of 1 K min−1. The jump in pressure at 271 K is caused by the complete decomposition of the hydrate. This abrupt release of all remaining gas slightly below the melting point of pure ice has also been found by other researchers.24–26 Subsequently, a decrease in pressure caused by the contraction of the sample due to melting and the dissolution of CO2 in water is visible from 273 K to 279 K. The relative mass of hydrate which is preserved until melting is only 3.5%. The preservation of such small amounts of hydrate for a very long time is observed in many of our own experiments and has also been reported in previous studies.24,25,29,34,38,53
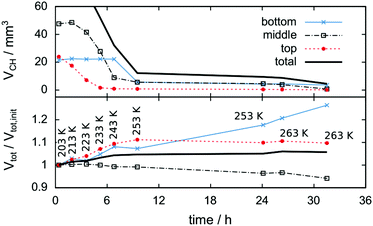
Fig. 6 shows tomograms of the sample at four different scan times at identical coordinates. The assignment of the different materials (hydrate, ice, or gas) to shades of gray is deduced empirically. Because of their respective theoretical densities and elemental compositions, among these materials, the hydrate should attenuate X-rays the strongest, followed by the ice, and finally, the gas. In general, the stronger the attenuation of X-rays, the brighter the gray scale values in our tomograms. Thus, dark shades of gray are assigned to gas, which attenuates the X-rays only weakly. The ice particles, produced by freezing water droplets in liquid nitrogen, are easily identifiable by their spherical shape and stability over time. The remaining shades of gray can be hydrate and decomposed hydrate only. Since the decomposed hydrate lacks CO2, it attenuates X-rays less than the intact hydrate does. In addition, regions of decomposed hydrate grow during the decomposition while regions of hydrate shrink. Histograms of regions r1, r2, and r3 (cf.Fig. 6, scan III) corresponding to decomposed hydrate, ice, and hydrate, respectively, and a line profile across all phases (cf.Fig. 6, line A-B-C-D, scan III) are shown in Fig. 7. Two important findings are apparent: (1) phases of ice, hydrate, decomposed hydrate, and gas are distinguishable by different gray scale values. Particularly, hydrate and decomposed hydrate show almost no overlap in their gray scale histograms. (2) The decomposed hydrate phase is slightly darker than the ice phase. Since both phases contain water molecules only, this implies that the apparent density of the decomposed hydrate is smaller than that of ice, i.e., the decomposed hydrate is porous. This rather good contrast helps with the segmentation of the raster image data, which in turn yields accurate volume information. While the accuracy and reliability of the volume information usually have to be estimated based on the image data alone, here we can make use of the independent pressure signal to verify our phase assignment. Fig. 8 displays the volume of decomposed hydrate as a function of mmin,CO2. The linear correlation between volume and mass is evident. The small offset of 0.6 mm3 in the fitting function V(m) = 0.6 + 5.1·m to the experimental data corresponds to the initial volume of hydrate, which decomposes during the loading procedure. All data points deviate from the linear fit by less than 4 mm3 (i.e., about 3% of the sample volume). This good correlation is found in all experiments of this work (cf. ESI†) and implies that our assignment of phases in the tomographic data is correct. Moreover, it justifies a more detailed local inspection of the sample based on the image data alone. This is done to examine the effect of the inhomogeneous temperature field on the hydrate stability. The volume containing the sample is subdivided into bottom, middle, and top regions. Each region has a height of 3 mm. The hydrate volume for each region is displayed as a function of time in the upper part of Fig. 9 together with the total hydrate volume. The top region of the hydrate decomposes within the first 5 h. During the same period, approximately 50% of the hydrate in the middle region decomposes while the bottom region shows no decomposition at all. Between 5 h and 10 h the hydrate in both the middle and the bottom regions decomposes almost completely. Only approximately 5 mm3 of hydrate remains in each of the two regions until the temperature is increased to 263 K. After 5 h at 263 K the rest of the hydrate of the middle region decomposes as well. The 5 mm3 of hydrate in the bottom region remains until the sample melts. The extraordinary stability of the bottom region during the first 7 h of the experiment can be explained using the non-uniform temperature field and its effects on the sample upon heating (cf. ESI,† Fig. S3). Table 2 provides the pair Tlow and Thigh,min as well as their difference ΔT. A comparison between the conditions at the start and end of each heating step (values in two successive lines in Table 2) shows that Tlow after heating is smaller than Thigh,min at the beginning of heating until Tlow is roughly 253 K. That implies that for Tlow < 253 K the coldest region of the sample (i.e., the bottom region) can never leave the hydrate stability region. The negative value for ΔT at Tlow = 263 K implies that the settled pressure (corresponding to Thigh,min) is below the V–I–H equilibrium pressure corresponding to Tlow. Since any temperature the sample is exposed to is above or equal to Tlow this means either that there is no hydrate left or that the absence or low rate of gas release prevented the attainment of the equilibrium pressure.
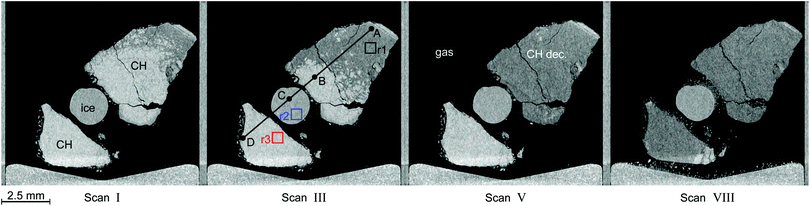 | ||
| Fig. 6 Tomograms of the sample configuration at four different times in a temperature stepping experiment. Roman numerals refer to the μCT scan number as in Fig. 5. In descending order of brightness the different regions are: clathrate hydrate (CH, white), ice (gray), decomposed hydrate (CH dec., dark gray), and gas (black). The squares in black (r1), blue (r2), and red (r3) in the tomogram of scan III denote regions used for the computation of histograms. They are depicted in Fig. 7 together with a line profile along the path from A to D. The wedge-shaped bright area at the bottom of the images is the cell base. | ||
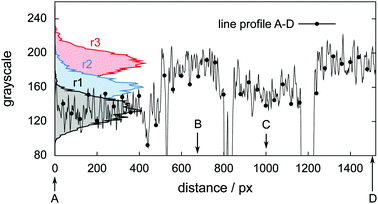 | ||
| Fig. 7 Histograms and line profile of gray scale values obtained from the tomogram of scan III shown in Fig. 6. The line profile starts in the decomposed hydrate region (point A), intersects with the hydrate region first (point B), the ice region afterwards (point C), and eventually ends in the hydrate region (point D). | ||
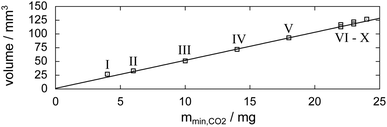 | ||
| Fig. 8 Relation between μCT image data and released gas mass mmin,CO2 (derived from the pressure signal). Markers denote the volume of the decomposed hydrate at the individual scan times (labeled by Roman numerals; cf.Fig. 5) as a function of the mass of released hydrate gas. The fitting function to the experimental data is V(m) = 0.6 + 5.1·m. | ||
| T low (K) | T high,min (K) | ΔT = Thigh,min − Tlow (K) |
|---|---|---|
| 203 | 220.5 | 17.5 |
| 213 | 229.5 | 16.5 |
| 223 | 237.8 | 14.8 |
| 233 | 245.7 | 12.7 |
| 243 | 252.3 | 9.3 |
| 253 | 257.6 | 4.6 |
| 263 | 256.2 | −6.8 |
The lower part of Fig. 9 tracks the sum of the hydrate and decomposed hydrate volumes over the experimental time relative to its value at the beginning of the experiment. A comparison between the total volume at the end and the beginning of the experiment yields a volumetric expansion of approximately 5% during hydrate decomposition. It is shown that the bottom region of the hydrate gains significantly more volume than the middle and top regions. The middle region is even losing some of its initial volume, although no displacement of the sample is observed. This can be explained by mass transport of water via the sublimation–diffusion–resublimation mechanism known from snow metamorphosis.67–70 Distinct indications for this are the needle like ice crystals growing in the interstices between the hydrate and the ice particles over the course of the experiment (cf.Fig. 6, scan VIII).
Eventually, during melting, the sum of the hydrate and decomposed hydrate volumes drops by 29% from solid to liquid. That is significantly larger than the volume reduction of pure ice during melting (9%). Similarly, a comparison of the volume of liquid water with the initial volume of the hydrate sample yields a volume reduction of 25%.
3.2 Isothermal experiments
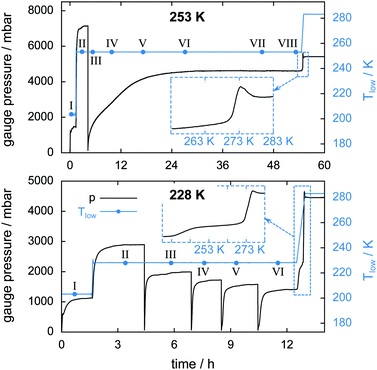
Two isothermal experiments, as described in Section 2.4 and Fig. 4, are carried out at Tlow = 228 K and Tlow = 253 K. The important key features of the experiments are listed in Table 1. An estimation of mmax(CO2) is omitted here due to the unknown amount of guest gas escaping the cell at rapid depressurization events. The pT-conditions during the experiments are displayed in Fig. 10 together with the starting times of the μCT scans. Four successive rapid depressurization events are triggered in the 228 K experiment while only one event is initiated at 253 K due to the small amount of hydrate left after the first opening. Due to very low dissociation rates in the 253 K experiment, μCT scans are possible even during decomposition (i.e., volume and phase changes over a 60 min long scan are negligible). A comparison between the pressure signals of the two isothermal experiments after the rapid depressurization procedure shows a distinct difference in the dissociation behaviour. While at 228 K the pressure settles within about 1 h after rapid depressurization it takes about 18 h to reach equilibrium at 253 K. This reduced rate of gas release at 253 K (with respect to 228 K) indicates that at 253 K the hydrate is self-preserved.Table 3 lists upper and lower bounds for the warmest sample temperature Thigh at the beginning and at the end of the isothermal phase of the experiment. It is assumed that initially the N2(g) partial pressure is 1 atm. With every cell opening event the N2(g) partial pressure is reduced. Hence, at the beginning of the experiment Thigh ≈ Thigh,min while at the end Thigh ≈ Thigh,max (cf. ESI†). A comparison between the sample temperature conditions (i.e., Tlow and Thigh) of the two isothermal experiments shows that for the 228 K experiment the temperature in the cell is nowhere above 253 K. Additionally, the temperature data indicate that the isothermal experiment at 253 K is done completely inside the self-preservation region (240 K < T < 273 K) while at 228 K the majority of the sample lies outside that region.
| Experiment | Scan no. | T high,min (K) | T high,max (K) |
|---|---|---|---|
| Isothermal 253 K | II | 262 | 265 |
| Isothermal 253 K | VI–VIII | 251 | 256 |
| Isothermal 228 K | II | 240 | 247 |
| Isothermal 228 K | VI | 225 | 236 |
The insets of Fig. 10 show the gauge pressure as a function of temperature during the final temperature ramp (2 K min−1) which leads to the complete decomposition of the hydrate samples. The characteristic pressure signal upon melting, already seen in Fig. 5, recurs. In both isothermal experiments the remaining gas is released shortly before melting of the ice starts. Notably, a flattening of the pressure curve is observed from about 240 K to 271 K in the 228 K isothermal experiment. Since a large amount of CO2 is released only upon melting this flattening is probably caused by the onset of self-preservation during heating.
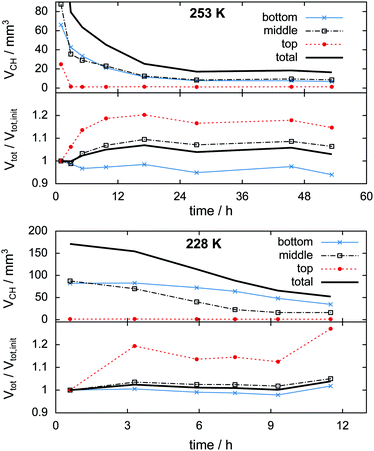
In the 253 K isothermal experiment (cf.Fig. 11, top) the top region of the sample decomposes almost completely before the cell is opened for pressure relief. A significant fraction of hydrate, totalling 20 mm3 or 10% of the initial sample volume, outlasts the rapid depressurization procedure in the middle and bottom regions. In contrast to the temperature stepping experiment, the hydrate volume in the middle and bottom regions here changes in an almost identical fashion after the cell opening event. This implies that rates of dissociation are similarly low in both regions. The first 17 h of decomposition are accompanied by a steady expansion of the sample region. The degree of expansion correlates with the ratio of decomposed to remaining hydrate. That means in relative numbers the top region grows more than the middle region, which grows more than the bottom region. Furthermore, the growth in the top region is observed mainly after 3 h when only a minute amount of hydrate is left. This implies that the expansion takes place primarily after decomposition. Subsequent to the period of expansion, the measured volumes stabilize and start to even shrink slightly. This can be explained using the growth of pores. Once pore sizes become big enough (>15 μm), void space becomes clearly distinguishable from solid phases, which results in a reduction of the measured solid volume.
In the isothermal experiment at 228 K (cf.Fig. 11, bottom) a similar behaviour is found in terms of expansion. The top region decomposes completely before the first scan is done and expands by 20% until the time of the second scan. The middle and bottom regions show almost no expansion during the first 9 h. Only at the end of the experiment does a total volumetric expansion of 3.6% become apparent. A reduction of the sample volume at advanced experimental time, as observed in the 253 K case, is missing here due to the much shorter duration of the experiment. Both the middle and the bottom regions contain an almost identical initial amount of approximately 85 mm3 hydrate. Contrary to the result of the isothermal experiment at 253 K, here the hydrate decomposes significantly faster in the middle region than in the bottom region. This behaviour reflects higher rates of dissociation attributed to the higher temperatures in the middle region.
3.3 Macroscopic defects and pore formation
In this section the focus is set on the macroscopic defects observed in the tomograms obtained from the μCT scans. No differences between the results obtained from the temperature stepping and the two isothermal experiments are found. Instead, a few common features, presented in Fig. 12–15, are observed in all three experiments. More views of μCT scans are provided in the ESI.†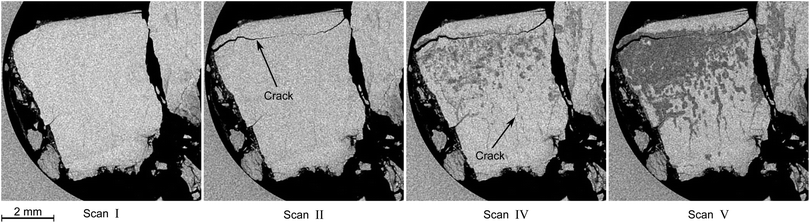 | ||
| Fig. 12 Typical degradation pattern as observed in horizontal tomograms obtained during the isothermal experiment at 228 K. Roman numerals refer to the scan numbers as labeled in Fig. 10. Hydrate is the bright, decomposed hydrate the dark gray, and gas the black phase. The circular region at the left side of the slices is the glassy carbon crucible. Note the appearance of a large crack in the tomogram of scan II and the subsequent advent of decomposition, which seems to start from the big crack initially and from smaller cracks at later times. | ||
 | ||
| Fig. 13 3D illustration of decomposing hydrate at four different stages of decomposition. The views are obtained from tomograms collected during the isothermal experiment at 228 K. Roman numerals refer to the scan numbers as labeled in Fig. 10. Regions of bright color represent hydrate and red regions correspond to already decomposed hydrate. | ||
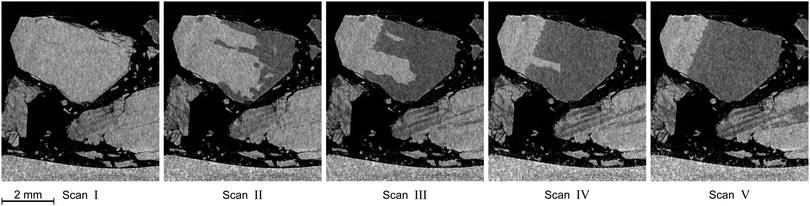 | ||
| Fig. 14 Distinctive degradation pattern as observed in vertical tomograms obtained during the isothermal experiment at 228 K. Roman numerals refer to the scan numbers marked in Fig. 10. Note how decomposition starts from the right side of the particle and stops at a plane which acts as a barrier for further decomposition. The blocking effect of that plane persists over the whole course of the experiment. | ||
 | ||
| Fig. 15 Horizontal tomograms, showing the process of pore formation taking place during the decomposition of a hydrate particle. The tomograms are obtained from the isothermal experiment at 253 K and labeled with Roman numerals according to the labeling in Fig. 10. Note how the particle expands as it decomposes (from left to right). The double headed arrow is provided as a visual aid for comparison. | ||
Similar observations were made by Takey et al.42,60 and Mimachi et al.61 in nondestructive studies of natural gas and methane hydrate pellets using phase contrast X-ray imaging. The authors speculated that differences in the thermal expansion between hexagonal ice (ice Ih) and hydrate (at 200 K the coefficient of linear expansion is a = 56 × 10−6 K−1 and c = 57 × 10−6 K−1 for hexagonal ice and 77 × 10−6 K−1 for sI hydrate)71 form thermal cracks upon cooling or heating of the hydrate. Note that the difference between the coefficients of thermal expansion of sI hydrate and ice Ih remains roughly the same at higher temperatures.72 The cracks are assumed to act as nucleation sites for hydrate decomposition. It is suggested that nucleation and the accompanying induction times at the crack are the rate-determining processes for hydrate decomposition after the initial step of dissociation at the free surface of the hydrate.60 In the present study, in most cases no sign of decomposition can be found in the vicinity of the crack at the time of crack appearance. Many cracks seem to form during the initial heating period from 203 K to the set point temperature. Additionally, in the samples used here roughly 20 wt% to 40 wt% of the water is presumably ice. Hence, differences in thermal expansion between ice and hydrate seem to be a plausible cause for the formation of cracks in this study as well. Nevertheless, mechanical stress caused by hydrate gas release or by a change in volume upon the hydrate–ice phase transformation can be an additional factor.
On the basis of Fig. 15 alone, we conclude that the macroscopic pores grow either from a network of pores already present in the hydrate samples or from pores produced during the decomposition process. In either case, the pore size of the initial pore network is well below the resolution limit of our μCT. However, porosities for our hydrate samples derived from the initial hydrate volume, hydrate composition and liquid water volume range from 4% to 24%. This implies that the hydrate samples are already porous after synthesis. Our data are in good agreement with the results of Kuhs et al.,55 who reported porosities ranging from 10% to 20% and pores with size ranging from 20 nm to 100 nm in CO2 hydrates produced by subjecting ice powder to pressurized gas. Even higher porosities of 44% were found by Circone et al.26 in CO2 hydrates produced from ice powder using a more complex temperature–pressure program. In general, the results from various studies show that hydrates of both natural and synthetic origin exhibit guest gas specific porosities, which are further correlated to the crystal structure, formation environment, and progress of decomposition.56–59,73–75 Here, the pores at the initial stage of the experiments are presumably a direct consequence of the sample preparation process involving ASW, which is known to be microporous.64,65,76
An increase of the apparent volume by more than 3% is found during hydrate decomposition in all of our μCT measurements and also observed in Fig. 15, where the diagonal of a hydrate particle grows by approximately 3% during decomposition. Melting at the end of the experiments leads to volume reductions between the solid and the liquid in the range of 27% to 38%. This is significantly larger than the volume reduction of dense ice during melting (9%). Additionally, during the phase transition from sI hydrate to ice, the water density increases from 0.79 g cm−3 (assuming a lattice constant of 12.0 Å) to 0.92 g cm−3. The growth of the apparent volume and the increase in the density of water together result in the occurrence of voids in the sample, which in turn leads to the large reduction of the apparent volume during melting. Since our results show only homogeneous regions of decomposed hydrate, no significant accumulation of void space or mass takes place during decomposition. Therefore, a homogeneous network of pores must have formed during decomposition. This agrees well with the SEM results of Stern et al.25 who reported an aerated appearance of ice as a hydrate dissociation product. Similarly, microstructures resembling Swiss cheese were found in decomposed natural gas75 and CO238 hydrates. Moreover, Takeya et al.31,49 reported extraordinarily large diffusivities of guest gas across the ice produced from hydrate decomposition and inferred diffusion across pores and along grain boundaries as a cause.
In this work, microporous regions of decomposed hydrate, once formed, grow during decomposition and spread out into the hydrate volume. The propagation front of decomposing regions differs widely and is found to be smooth in some and highly irregular in other cases. These characteristics of the propagation front are likely determined by the microstructure before decomposition.
The thickness of porous ice as a decomposition product is of minor importance with respect to its ability to form an efficient diffusion barrier, especially if the pore network is permeable. On the other hand, it is conceivable that water vapor generated by decomposing hydrates can under certain conditions plug such a network of pores by deposition at the pore walls. No matter whether the pore network is connected or not, due to the small pore size, plugging could eventually yield a highly pressure resistant structure that can explain how a rather thin layer of ice can withstand the pressures developing in self-preserved hydrates far outside their stability region.
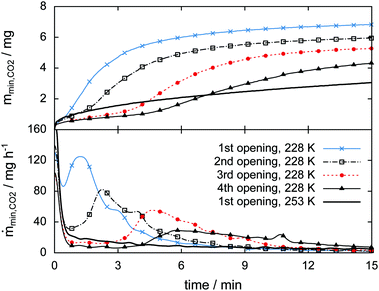
3.4 Rapid depressurization events
The high sampling rate of the pT-data acquisition combined with the high accuracy of the pressure signal is used for a detailed analysis of the rapid depressurization events. Fig. 16 shows the mass of the gas released and its rate as a function of time immediately after the cell is closed. In the isothermal experiment at 253 K the gas release seems to follow a two-step process. The first step is characterized by a vigorous release of gas at a release rate of 170 mg h−1. Within one minute after rapid depressurization the gas release rate drops to below 20 mg h−1 and decreases steadily henceforth. This low rate of gas release marks the second step of the process. Both steps were also reported by Handa47 and verified in various studies later on.31,33,49,77Similarly high gas release rates of more than 100 mg h−1 and a subsequent drop of rates are also observed every time after rapid depressurization in the 228 K isothermal experiment. However, at 228 K a third step, characterized by a pronounced period of gas release, is observed at later times. With an increasing number of rapid depressurization events this third step happens at later times and with decreasing strength.
Since this behavior is unexpected, this part of the experiment is repeated several times using additional samples. The focus is set on rapid depressurization events at 228 K and 253 K only. μCT scans are used to determine volume and phase information. The two-step process of gas release observed at 253 K recurs in all of six additional rapid depressurization events performed with three samples. Remarkably, the third step of gas release is confirmed in only one out of 14 additional rapid depressurization events accomplished using four different samples at 228 K. Agreement is found in the case of a sample with a molar ratio CO2·nH2O of n = 13.3. In the remaining 13 events the hydrate is of even lower quality (n > 13.7). Notably, all of these 13 events show some degree of self-preservation (even at 228 K), expressed by remarkably low gas release rates. This is most pronounced in the case of maximal molar water content (n = 30) and is shown in Fig. S11 of the ESI.†
It is remarkable that the initial two steps of hydrate decomposition are the same for the self-preserved (228 K) and the non-preserved (253 K) hydrate. Nevertheless, it is unclear what causes this second phase (i.e., the third step) of strong gas release. A possible explanation is the sudden occurrence of new decomposition sites. These could be produced by the formation of new cracks or the bursting of existing decomposition barriers. For now, our results are not able to confirm or exclude any of these suggestions.
4 Conclusions
Establishing a predictive model for the decomposition of clathrate hydrates is a worthwhile but difficult task. It is worthwhile because such a model will eventually help with the estimation of the applicability of hydrate technologies. It is difficult because such a model has to combine thermodynamics, decomposition kinetics, heat and mass transport mechanisms as well as micro- and macroscopic features of the bulk and surface. Similarly, the isolation of a single parameter of decomposition is very challenging in experiments. Thus, until today there is no comprehensive model which can be used to explain the decomposition behavior of hydrates in its entirety.In this work, we studied the influence of micro- and macroscopic features on the decomposition behavior of clathrate hydrates. Particularly, the role of cracks and pores is addressed using μCT in combination with accurate temperature control and pressure monitoring. The good contrast between hydrate and decomposed hydrate is used to segment the tomographic data set into regions of different phases. The assignment of these phases is very accurate, as verified using released gas mass data obtained from the pressure signal. This cross-correlation, which is shown for the first time, allows for a detailed study of the structural information obtained from μCT. So far, the experimental setup used is not capable of resolving either thin layers (<10 μm) of ice or small density differences between supercooled liquid water and ice. Hence, the direct examination of popular self-preservation hypotheses, such as a thin diffusion limiting ice-shield37,38 or liquid water as an intermediate decomposition product,27,28,54 is difficult. Nevertheless, the observations made here add new perspectives to the understanding of decomposition and self-preservation of CO2 hydrates. Besides a homogeneous network of pores, additional features like cracks and decomposition barriers form in our samples during hydrate decomposition.
Since the apparent particle volume does not reduce during hydrate decomposition, the formation of porous ice must be a consequence of the increase in water density from hydrate (0.79 g cm−3) to ice (0.92 g cm−3). Depending on the pore size distribution and the pore connectivity, a network of pores can offer many possible pathways for the gas to escape. In the extreme cases, this can range from a gas-tight layer of closed pores to a permeable network of open pores.
Although the formation of cracks during hydrate decomposition has been observed earlier,42,60,61 their role in the decomposition process of hydrates is rarely discussed. Since our results show that cracks form very often and always act as a precursor for hydrate decomposition, their role seems to be important for macroscopic samples like ours. It is furthermore conceivable that cracks, or actually, the absence of cracks, are an important ingredient regarding the phenomenon of self-preservation. That is because any formation of cracks will result in new decomposition sites resulting in the release of gas. While mechanical stress induced by hydrate decomposition can cause the formation of cracks, our observations also support the notion that the formation of cracks is related to differences between the thermal expansion of ice and hydrate. Local changes in temperature at interfaces between hydrate and ice, caused by either endothermic cooling upon decomposition or external heat, can lead to small cracks and the formation of new decomposition sites. The gas released from these sites can in turn result in mechanical stress and the enlargement of the cracks. This process can be used to explain the second phase of strong gas release observed in our rapid depressurization experiments at 228 K.
Decomposition barriers, a complex and irregular decomposition front, and seemingly resistant regions to decomposition at the exposed sample surface are found at temperatures inside and outside the self-preservation regime. These results do not match the shrinking-core model of hydrate decomposition in which decomposition is assumed to start from the exposed hydrate surface, proceeding inward.31,49 Similarly, our results do not support the theory of an ice-shield protecting the hydrate from further decomposition, as is often assumed to explain the phenomenon of self-preservation. However, our observations that some connected regions of the hydrate (in contact with the free surface) decompose while similar regions remain intact point towards a local cause of the decomposition process. Since a non-uniform distribution of the hydration number is a natural consequence of the gas-phase deposition process used to make such samples, we infer that the local distribution of guest molecules and ice, i.e., the hydration number, plays an important role in the decomposition process.
Altogether our results show a variety of phenomena associated with hydrate decomposition in macroscopically-sized samples. Further analysis is needed to study the role of sample preparation as well as the influence of parameters like temperature, pressure, particle size, and ice content on the cracks and barriers on both micro- and macroscopic scales. Nevertheless, it is established that pores, cracks, and decomposition barriers are important factors in hydrate decomposition and the self-preservation phenomenon.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Stefan Arzbacher, Nima Rahmatian, and Jörg Petrasch thank the Austrian Research Promotion Agency (FFG) for financial support (TomoFuma project, no. 839070). Thomas Loerting received funding from the Austrian Science Fund (FWF) within the project no. I-1392.References
- E. D. Sloan and C. Koh, Clathrate Hydrates of Natural Gases, CRC Press, 3rd edn, 2007 Search PubMed.
- Gas Hydrates 1: Fundamentals, Characterization and Modeling, ed. D. Broseta, L. Ruffine and A. Desmedt, ISTE Ltd/John Wiley and Sons Inc, Hoboken, NJ, 2017 Search PubMed.
- Gas Hydrates 2: Geoscience Issues and Potential Industrial Applications, ed. L. Ruffine, D. Broseta and A. Desmedt, ISTE Ltd/John Wiley and Sons Inc, Hoboken, NJ, 2018 Search PubMed.
- M. von Stackelberg and H. R. Müller, Z. Electrochem., 1954, 58, 25–39 Search PubMed.
- H. M. Barnes, L. N. Kremzner and W. A. Mitchell, US Pat., US2975603A, General Foods Corp., 1958 Search PubMed.
- A. Aldred, R. D. Bee, V. S. E. Evans and M. Trycholik-Kinavuidi, WO Pat., WO2017093084A1, Unilever Plc, 2015 Search PubMed.
- T. Hatakeyama, E. Aida, T. Yokomori, R. Ohmura and T. Ueda, Ind. Eng. Chem. Res., 2009, 48, 4083–4087 CrossRef CAS.
- G. K. Anderson, J. Chem. Thermodyn., 2003, 35, 1171–1183 CrossRef CAS.
- L. Fournaison, A. Delahaye, I. Chatti and J.-P. Petitet, Ind. Eng. Chem. Res., 2004, 43, 6521–6526 CrossRef CAS.
- W. Zhang, Y. Wang, X. Lang and S. Fan, Energy Convers. Manage., 2017, 146, 43–51 CrossRef CAS.
- Z. Ma, P. Zhang, H. Bao and S. Deng, Renewable Sustainable Energy Rev., 2016, 53, 1273–1302 CrossRef CAS.
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- P. Babu, P. Linga, R. Kumar and P. Englezos, Energy, 2015, 85, 261–279 CrossRef CAS.
- H. Dashti, L. Zhehao Yew and X. Lou, J. Nat. Gas Sci. Eng., 2015, 23, 195–207 CrossRef CAS.
- S. M. Benson and F. M. Orr, MRS Bull., 2008, 33, 303–305 CrossRef CAS.
- H. Sakai, T. Gamo, E.-S. Kim, M. Tsutsumi, T. Tanaka, J. Ishibashi, H. Wakita, M. Yamano and T. Oomori, Science, 1990, 248, 1093–1096 CrossRef CAS PubMed.
- O. Prieto-Ballesteros, J. S. Kargel, M. Fernandez-Sampedro, F. Selsis, E. S. Martinez and D. L. Hogenboom, Icarus, 2005, 177, 491–505 CrossRef CAS.
- S. W. Kieffer, X. Lu, C. M. Bethke, J. R. Spencer, S. Marshak and A. Navrotsky, Science, 2006, 314, 1764–1766 CrossRef CAS PubMed.
- D. P. Cruikshank, A. W. Meyer, R. H. Brown, R. N. Clark, R. Jaumann, K. Stephan, C. A. Hibbitts, S. A. Sandford, R. M. Mastrapa, G. Filacchione, C. M. D. Ore, P. D. Nicholson, B. J. Buratti, T. B. McCord, R. M. Nelson, J. B. Dalton, K. H. Baines and D. L. Matson, Icarus, 2010, 206, 561–572 CrossRef CAS.
- A.-H. Delsemme and P. Swings, Ann. Astrophys., 1952, 15, 1–6 CAS.
- S. L. Miller, Proc. Natl. Acad. Sci. U. S. A., 1961, 47, 1798–1808 CrossRef CAS.
- S. L. Miller and W. D. Smythe, Science, 1970, 170, 531–533 CrossRef CAS PubMed.
- J. A. Ripmeester and S. Alavi, Curr. Opin. Solid State Mater. Sci., 2016, 20, 344–351 CrossRef CAS.
- L. A. Stern, S. Circone, S. H. Kirby and W. B. Durham, J. Phys. Chem. B, 2001, 105, 1756–1762 CrossRef CAS.
- L. A. Stern, S. Circone, S. H. Kirby and W. B. Durham, Can. J. Phys., 2003, 81, 271–283 CrossRef CAS.
- S. Circone, L. A. Stern, S. H. Kirby, W. B. Durham, B. C. Chakoumakos, C. J. Rawn, A. J. Rondinone and Y. Ishii, J. Phys. Chem. B, 2003, 107, 5529–5539 CrossRef CAS.
- V. Melnikov, A. Nesterov, A. Reshetnikov and A. Zavodovsky, Chem. Eng. Sci., 2009, 64, 1160–1166 CrossRef CAS.
- V. Melnikov, A. Nesterov, A. Reshetnikov, V. Istomin and V. Kwon, Chem. Eng. Sci., 2010, 65, 906–914 CrossRef CAS.
- A. Falenty, W. F. Kuhs, M. Glockzin and G. Rehder, Energy Fuels, 2014, 28, 6275–6283 CrossRef CAS.
- A. S. Stoporev, A. Y. Manakov, L. K. Altunina, A. V. Bogoslovsky, L. A. Strelets and E. Y. Aladko, Energy Fuels, 2014, 28, 794–802 CrossRef CAS.
- S. Takeya, T. Ebinuma, T. Uchida, J. Nagao and H. Narita, J. Cryst. Growth, 2002, 237–239, 379–382 CrossRef CAS.
- S. Takeya, T. Uchida, J. Nagao, R. Ohmura, W. Shimada, Y. Kamata, T. Ebinuma and H. Narita, Chem. Eng. Sci., 2005, 60, 1383–1387 CrossRef CAS.
- S. Takeya and J. A. Ripmeester, Angew. Chem., Int. Ed., 2008, 120, 1296–1299 CrossRef.
- S. Takeya and J. A. Ripmeester, ChemPhysChem, 2010, 11, 70–73 CrossRef CAS PubMed.
- H. Mimachi, S. Takeya, A. Yoneyama, K. Hyodo, T. Takeda, Y. Gotoh and T. Murayama, Chem. Eng. Sci., 2014, 118, 208–213 CrossRef CAS.
- S. Takeya, S. Muromachi, Y. Yamamoto, H. Umeda and S. Matsuo, Fluid Phase Equilib., 2016, 413, 137–141 CrossRef CAS.
- W. F. Kuhs, G. Genov, D. K. Staykova and T. Hansen, Phys. Chem. Chem. Phys., 2004, 6, 4917–4920 RSC.
- A. Falenty and W. F. Kuhs, J. Phys. Chem. B, 2009, 113, 15975–15988 CrossRef CAS PubMed.
- H. Ohno, H. Narita and J. Nagao, J. Phys. Chem. Lett., 2011, 2, 201–205 CrossRef CAS.
- L. Yang, A. Falenty, M. Chaouachi, D. Haberthür and W. F. Kuhs, Geochem., Geophys., Geosyst., 2016, 3717–3732 CrossRef CAS.
- S. Takeya, K. Honda, Y. Gotoh, A. Yoneyama, K. Ueda, A. Miyamoto, T. Hondoh, A. Hori, D. Sun, R. Ohmura, K. Hyodo and T. Takeda, J. Synchrotron Radiat., 2012, 19, 1038–1042 CrossRef CAS PubMed.
- S. Takeya, A. Yoneyama, K. Ueda, K. Hyodo, T. Takeda, H. Mimachi, M. Takahashi, T. Iwasaki, K. Sano, H. Yamawaki and Y. Gotoh, J. Phys. Chem. C, 2011, 115, 16193–16199 CrossRef CAS.
- W. Shimada, S. Takeya, Y. Kamata, T. Uchida, J. Nagao, T. Ebinuma and H. Narita, J. Phys. Chem. B, 2005, 109, 5802–5807 CrossRef CAS PubMed.
- V. Buch, J. P. Devlin, I. A. Monreal, B. Jagoda-Cwiklik, N. Uras-Aytemiz and L. Cwiklik, Phys. Chem. Chem. Phys., 2009, 11, 10245–10265 RSC.
- J.-R. Zhong, X.-Y. Zeng, F.-H. Zhou, Q.-D. Ran, C.-Y. Sun, R.-Q. Zhong, L.-Y. Yang, G.-J. Chen and C. A. Koh, Sci. Rep., 2016, 6, 38855 CrossRef CAS.
- D. Davidson, S. Garg, S. Gough, Y. Handa, C. Ratcliffe, J. Ripmeester, J. Tse and W. Lawson, Geochim. Cosmochim. Acta, 1986, 50, 619–623 CrossRef CAS.
- Y. Handa, J. Chem. Thermodyn., 1986, 18, 891–902 CrossRef CAS.
- V. S. Yakushev and V. Istomin, Physics and Chemistry of Ice, Sapporo, Japan, 1992, pp. 136–140 Search PubMed.
- S. Takeya, W. Shimada, Y. Kamata, T. Ebinuma, T. Uchida, J. Nagao and H. Narita, J. Phys. Chem. A, 2001, 105, 9756–9759 CrossRef CAS.
- V. A. Istomin, V. S. Yakushev, N. A. Makhonina, V. G. Kwon and E. M. Chuvilin, Gas Ind. Russ., 2006, 4, 16–27 Search PubMed.
- V. P. Melnikov, L. S. Podenko, A. N. Nesterov, A. O. Drachuk, N. S. Molokitina and A. M. Reshetnikov, Dokl. Chem., 2016, 466, 53–56 CrossRef CAS.
- J. W. Wilder and D. H. Smith, J. Phys. Chem. B, 2002, 106, 226–227 CrossRef CAS.
- L. A. Stern, S. Circone, S. H. Kirby and W. B. Durham, J. Phys. Chem. B, 2002, 106, 228–230 CrossRef CAS.
- V. Melnikov, A. Nesterov, A. Reshetnikov and V. Istomin, Chem. Eng. Sci., 2011, 66, 73–77 CrossRef CAS.
- W. F. Kuhs, A. Klapproth, F. Gotthardt, K. Techmer and T. Heinrichs, Geophys. Res. Lett., 2000, 27, 2929–2932 CrossRef CAS.
- L. A. Stern, S. H. Kirby, S. Circone and W. B. Durham, Am. Mineral., 2004, 89, 1162–1175 CrossRef CAS.
- G. Bohrmann, W. F. Kuhs, S. A. Klapp, K. S. Techmer, H. Klein, M. M. Murshed and F. Abegg, Mar. Geol., 2007, 244, 1–14 CrossRef.
- S. A. Klapp, G. Bohrmann, W. F. Kuhs, M. M. Murshed, T. Pape, H. Klein, K. S. Techmer, K. U. Heeschen and F. Abegg, Mar. Pet. Geol., 2010, 27, 116–125 CrossRef CAS.
- S. A. Klapp, F. Enzmann, P. Walz, T. Huthwelker, J. Tuckermann, J.-O. Schwarz, T. Pape, E. T. Peltzer, R. Mokso, D. Wangner, F. Marone, M. Kersten, G. Bohrmann, W. F. Kuhs, M. Stampanoni and P. G. Brewer, Geo-Mar. Lett., 2012, 32, 555–562 CrossRef CAS.
- S. Takeya, A. Yoneyama, K. Ueda, H. Mimachi, M. Takahashi, K. Sano, K. Hyodo, T. Takeda and Y. Gotoh, J. Phys. Chem. C, 2012, 116, 13842–13848 CrossRef CAS.
- H. Mimachi, M. Takahashi, S. Takeya, Y. Gotoh, A. Yoneyama, K. Hyodo, T. Takeda and T. Murayama, Energy Fuels, 2015, 29, 4827–4834 CrossRef CAS.
- C. Mitterdorfer, M. Bauer and T. Loerting, Phys. Chem. Chem. Phys., 2011, 13, 19765 RSC.
- S. Arzbacher, J. Petrasch, A. Ostermann and T. Loerting, Materials, 2016, 9, 668 CrossRef PubMed.
- E. Mayer and A. Hallbrucker, J. Chem. Soc., Chem. Commun., 1989, 749–751 RSC.
- A. Hallbrucker and E. Mayer, J. Chem. Soc., Faraday Trans., 1990, 86, 3785–3792 RSC.
- L. Grady, IEEE Trans. Pattern Anal. Mach. Intell., 2006, 28, 1768–1783 Search PubMed.
- Z. Yosida, H. Oura, D. Kuroiwa, T. Huzioka, K. Kojima, S.-I. Aoki and S. Kinosita, Contributions from the Institute of Low Temperature Science, 1955, vol. 7, pp. 19–74 Search PubMed.
- B. R. Pinzer, M. Schneebeli and T. U. Kaempfer, The Cryosphere, 2012, 6, 1141–1155 CrossRef.
- K. Hammonds, R. Lieb-Lappen, I. Baker and X. Wang, Cold Reg. Sci. Technol., 2015, 120, 157–167 CrossRef.
- K. Hammonds and I. Baker, Cold Reg. Sci. Technol., 2016, 125, 12–20 CrossRef.
- J. S. Tse, J. Inclusion Phenom. Mol. Recognit. Chem., 1990, 8, 25–32 CrossRef CAS.
- K. C. Hester, Z. Huo, A. L. Ballard, C. A. Koh, K. T. Miller and E. D. Sloan, J. Phys. Chem. B, 2007, 111, 8830–8835 CrossRef CAS PubMed.
- D. K. Staykova, W. F. Kuhs, A. N. Salamatin and T. Hansen, J. Phys. Chem. B, 2003, 107, 10299–10311 CrossRef CAS.
- G. Genov, W. F. Kuhs, D. K. Staykova, E. Goreshnik and A. N. Salamatin, Am. Mineral., 2004, 89, 1228–1239 CrossRef CAS.
- W. F. Kuhs, G. Genov, E. Goreshnik, A. Zeller, K. S. Techmer and G. Bohrmann, Int. J. Offshore Polar Eng., 2004, 14, 305–309 CAS.
- C. R. Hill, C. Mitterdorfer, T. G. A. Youngs, D. T. Bowron, H. J. Fraser and T. Loerting, Phys. Rev. Lett., 2016, 116, 215501 CrossRef PubMed.
- S. Takeya and J. A. Ripmeester, Proceedings of the 7th International Conference on Gas Hydrates (ICGH 2011), Edinburgh, Scotland, UK, 2011.
Footnote |
| † Electronic supplementary information (ESI) available: Section 1: Tomographic setup. Section 2: Temperature calibration. Section 3: Temperature field. Section 4: Leakage. Section 5: Mass calibration. Section 6: Image processing. Section 7: 3D illustrations of CT results and pressure–temperature data of a verification experiment. See DOI: 10.1039/c8cp07871h |
| This journal is © the Owner Societies 2019 |