Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spectroscopic characterisation of centropolyindanes†
Stewart F.
Parker
 *a,
Lisha
Zhong
*a,
Lisha
Zhong
 b,
Marco
Harig
c and
Dietmar
Kuck
b,
Marco
Harig
c and
Dietmar
Kuck
 c
c
aISIS Facility, STFC Rutherford Appleton Laboratory, Chilton, Didcot, OX11 0QX, UK. E-mail: stewart.parker@stfc.ac.uk
bDowning College, University of Cambridge, Regent Street, Cambridge, CB2 1DQ, UK
cDepartment of Chemistry and Center for Molecular Materials (CM2), Bielefeld University, 33615 Bielefeld, Germany
First published on 4th February 2019
Abstract
A highly promising class of three-dimensional polyaromatic hydrocarbons comprises the centropolyindanes. The characteristic feature of these compounds is the mutual fusion of several molecules of indane along the saturated C–C bonds of their cyclopentane rings. Among the polycyclic aromatic hydrocarbons, the centropolyindanes are special because of the saturated core of sp3-hybridised carbon atoms embedded in a three-dimensional environment of aromatic building blocks. While the centropolyindanes and their numerous derivatives have been studied in detail by NMR spectroscopy, mass spectrometry and X-ray diffraction, investigation of their vibrational features, and especially those of the neopentane core present in most cases, have not been performed so far. In the present paper, we report the first systematic study of a set of centropolyindanes by vibrational spectroscopy, using inelastic neutron scattering (INS), infrared and Raman spectroscopies.
Introduction
In the past decades, the combination of the fundamental structural tool-kit of organic chemistry with geometrically strict motifs has led to an enormous expansion of non-natural molecular architecture that represents a classical field of chemical research.1 This holds true in particular for ideally two-dimensional carbon frameworks based on hexagonal benzene building blocks2,3 as well as for the distorted variants, most of which contain non-hexagonal, such as five-, seven- and eight-membered ring elements,4–9 and includes tube-like10 and fully globular carbon networks.11 Other albeit less variable molecular motifs comprise linear, acetylene- and cumulene-based carbon chains.12 Strictly three-dimensional carbon-based constructs have remained relatively scarce but have started to gain increasing attention.13–17 In this context, a highly promising class of novel three-dimensional molecular motifs comprises the centropolyindanes.18The characteristic feature of the centropolyindane hydrocarbons is the mutual fusion of several molecules of indane (1, Chart 1) along the saturated C–C bonds of their five-membered (cyclopentane) rings. For example, several centrotriindanes are known, such as triptindane, 2 (a tribenzo[3.3.3]propellane or “monofuso-centrotriindane”),13,19,20 the angularly fused difuso-centrotriindane 4,21 and tribenzotriquinacene 3 (a trifuso-centrotriindane).22,23 Fenestrindane, 6,24 represents one of the two possible regular centrotetraindanes.18 As an extreme, six indane units can be merged about a common neopentane core, generating centrohexaindane, which has been addressed as a “Cartesian” hydrocarbon due to the strictly orthogonal orientation of its six benzene units in space.25 In recent years, decisive progress has been made to merge the particular structural motifs of tribenzotriquinacene (3) and fenestrindane (6) with genuine benzenoid frameworks.26,27
 | ||
| Chart 1 Structures of the compounds studied. Below the sample numbers, the chemical name, the empirical formula and the molecular point group are given. If the solid-state structure has been determined, the space group, followed by the Cambridge Structural Database29 refcode and the reference to the structure are also presented. Ball-and-stick representations of the structures are given in Fig. S1–S6, S13, S15, S17 and S19 of the ESI.† | ||
Among the polycyclic aromatic hydrocarbons, the centropolyindanes are special because of the saturated, sp3-hybridised core embedded in a three-dimensional environment of aromatic building blocks. While these three-dimensional hydrocarbons and their numerous derivatives have been studied in detail by NMR spectroscopy, mass spectrometry and X-ray diffraction,28 investigation of their vibrational features, and especially those of the neopentane core, have not been performed so far. In the present paper, we report the first systematic study of a set of centropolyindanes by vibrational spectroscopy, using inelastic neutron scattering (INS), infrared and Raman spectroscopies. The nine centropolyindanes, 2–10, investigated here are displayed in Chart 1, along with their structural parent hydrocarbon, indane (1).
Experimental
Materials
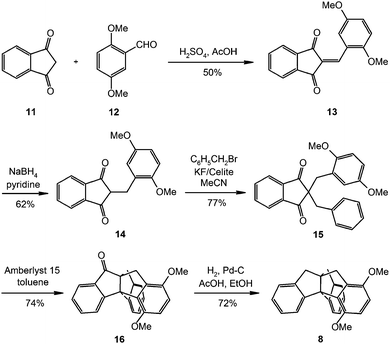
Indane (Aldrich, 95%) was used as received. Triptindane (2),20 tribenzotriquinacene (3),22 difuso-centrotriindane (4),21 tribenzo[5.5.5.5]fenestrene (5),24 fenestrindane (6),24 triptindan-9-one (7),20 12d-methyltribenzotriquinacene (9)21 and 4b,8b,12b,12d-tetramethyltribenzotriquinacene (10)30 were synthesised as described in the literature.18 The synthesis of 1,4-dimethoxytriptindane (8) was performed as shown in Scheme 1. The details are given in the ESI.†Vibrational spectroscopy
Inelastic neutron scattering (INS) spectra were recorded at less than 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K using TOSCA31 at ISIS (http://www.isis.stfc.ac.uk/). The spectra are available at the INS database: http://wwwisis2.isis.rl.ac.uk/INSdatabase/. Infrared spectra were recorded using a Bruker Vertex70 FTIR spectrometer, over the range 100 to 4000
K using TOSCA31 at ISIS (http://www.isis.stfc.ac.uk/). The spectra are available at the INS database: http://wwwisis2.isis.rl.ac.uk/INSdatabase/. Infrared spectra were recorded using a Bruker Vertex70 FTIR spectrometer, over the range 100 to 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1 at 4 cm−1 resolution with a DLaTGS detector using 64 scans and the Bruker Diamond ATR. Variable temperature infrared spectra were recorded for some samples using a Specac Golden Gate accessory. The use of the ultra-wide range beam splitter enabled the entire spectral range to be recorded without the need to change beam splitters. The spectra have been corrected for the wavelength-dependent variation in path length using the Bruker software. FT-Raman spectra were recorded with a Bruker MultiRam spectrometer using 1064
cm−1 at 4 cm−1 resolution with a DLaTGS detector using 64 scans and the Bruker Diamond ATR. Variable temperature infrared spectra were recorded for some samples using a Specac Golden Gate accessory. The use of the ultra-wide range beam splitter enabled the entire spectral range to be recorded without the need to change beam splitters. The spectra have been corrected for the wavelength-dependent variation in path length using the Bruker software. FT-Raman spectra were recorded with a Bruker MultiRam spectrometer using 1064![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm excitation, 4
nm excitation, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1 resolution, 500
cm−1 resolution, 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mW laser power and 64 scans. Variable temperature Raman spectra were recorded using 785 nm excitation and a Renishaw InVia spectrometer that has been previously described.32 For both Raman instruments, the low wavenumber limit was ∼50 cm−1, as determined by the laser line rejection filter.
mW laser power and 64 scans. Variable temperature Raman spectra were recorded using 785 nm excitation and a Renishaw InVia spectrometer that has been previously described.32 For both Raman instruments, the low wavenumber limit was ∼50 cm−1, as determined by the laser line rejection filter.
Computational studies
Isolated molecule calculations were carried out with Gaussian 03 and Gaussian 09.33 In both cases, the 6-311G(d) basis set was used with the B3LYP hybrid functional. Solid state calculations used the plane wave pseudopotential-based program CASTEP34,35 with the inclusion of the Tkatchenko and Scheffler36 dispersion correction for the calculation of the vibrational transition energies and their intensities. The generalized gradient approximation Perdew–Burke–Ernzerhof functional was used in conjunction with optimized norm-conserving pseudopotentials. All of the calculations were converged to better than |0.0035| eV Å−1. After geometry optimization, the vibrational spectra were calculated in the harmonic approximation using density functional perturbation theory. Both Gaussian and CASTEP generate the vibrational eigenvalues and eigenvectors, which allows visualization of the modes within GaussView37 and Materials Studio (http://accelrys.com/products/collaborative-science/biovia-materials-studio/) respectively. It is also the information needed to calculate the INS spectrum using the program ACLIMAX.38 Infrared and Raman spectra were generated from the Gaussian output using either GaussView or GaussSum.39 We emphasize that the transition energies have not been scaled.Results and discussion
The structures of the compounds studied are shown in Chart 1. Ball-and-stick representations are given in Fig. S1–S6, S13, S15, S17 and S19 of the ESI.† For some of the compounds, the structure is known from X-ray crystallography (2,403,41624 and 930,41), the remainder have been characterised by solution NMR spectroscopy and mass spectrometry. This group of compounds was chosen so as to examine: (i) how the progressive addition of indane (1) sub-units to the core (2, 3, 4, 5, 6) and (ii) substitution of the indane framework (2, 7, 8) and substitution at the central carbons (3, 9, 10) modifies the spectra.Fig. 1–3 show the INS, infrared and FT-Raman spectra of a series of compounds in order of increasing complexity (1, 2, 3, 4, 5, 6). The three types of spectra for each compound are shown in Fig. S1–S6 (ESI†).
 | ||
| Fig. 1 INS spectra from top to bottom of: solid indane (1), triptindane (2), tribenzotriquinacene (3), difuso-centrotriindane (4), tribenzo[5.5.5.5]fenestrene (5) and fenestrindane (6). | ||
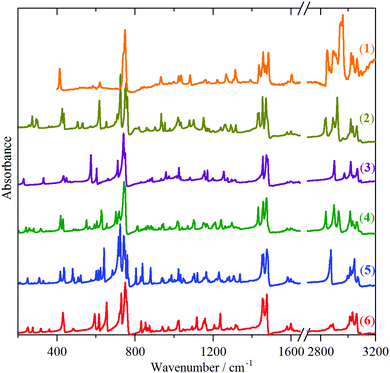 | ||
| Fig. 2 Infrared spectra from top to bottom of: solid indane (1), triptindane (2), tribenzotriquinacene (3), difuso-centrotriindane (4), tribenzo[5.5.5.5]fenestrene (5) and fenestrindane (6). | ||
While the spectra are clearly very rich, each technique shows a remarkable similarity between the different molecules. In the INS spectra (Fig. 1), the overall pattern is similar in the 400–1600 cm−1 range, in the infrared spectra (Fig. 2), strong bands are seen at ∼450, 750 and 1480 cm−1, and in the Raman spectra (Fig. 3), strong modes occur at ∼150, 750, 1020 and 1600 cm−1. This suggests that the vibrational spectra are largely characteristic of indane. While it is perhaps unsurprising that the aromatic modes are largely unchanged, the very different central framework of the molecules might have been expected to be reflected in the spectra. The approach that has been adopted was to carry out a detailed assignment of the parent molecule, indane (1), and then to examine the differences between the systems.
Indane (1)
Indane (1) has two possible conformations of the C5 ring: planar (C2v) and buckled (Cs). It is formally a cyclopentene derivative and the parent molecule is known to be puckered,42,43i.e., to have Cs symmetry. Further, while the crystal structure of indane itself is not known, that of many of its derivatives are known and these all exhibit a puckered C5 ring. For example, in 4- and 5-nitroindane44 the CH2 group is 5.0° and 12.7° out of the ring and in trans,trans-1,2,3-triphenylindane45 it is 16.9° out of the ring. An isolated molecule, i.e. gas phase, calculation of the planar C2v structure found one imaginary mode that corresponded to movement of the CH2 group out-of-the plane. Comparison of the calculated isolated molecule buckled (Cs) structure with the gas phase structure46 shows good agreement.Fig. 4 compares the experimental solid state INS spectrum and the calculated isolated (i.e. gas phase) molecule INS spectrum of indane. The isolated molecule does not include the lattice modes (the intense broad band centered at ∼100 cm−1 in Fig. 4a) but in the internal mode region above 150 cm−1, it can be seen that the agreement is excellent. Fig. S7 (ESI†) shows similarly good agreement for the infrared spectra, with the exception of the C–H stretch modes which are, as is the usual case, calculated at too high an energy. This is because the calculation assumes a harmonic potential and the real system is anharmonic.47
Spectroscopic studies of indane have been dominated by attempts to determine the puckering potential in the gas phase.48–50 The infrared and Raman spectra and their assignment for the liquid phase have been reported;51 there are no data for the solid state. Table S1 (ESI†) lists the observed and calculated transition energies and their assignment. These are largely in agreement with the only previous work,51 which was based on an empirical force field. The disagreements are mainly in the low energy region, <500 cm−1, where the infrared and Raman bands are weak, whereas the deformations result in large amplitude motions of the hydrogen atoms and hence strong INS modes. It is noteworthy that the transition energies of the phenylene (C6H4) modes are typical of those found for ortho-disubstituted benzenes.52,53
Comparison of the spectra
As crystal structures are not available for the whole set of the compounds, we anticipated that in the absence of significant intermolecular interactions, the isolated molecule approximation should provide a good description of the vibrational spectra. To enable comparisons, this was used for all of the molecules. Tribenzotriquinacene (3) is the simplest of the centropolyindanes studied here, consisting of three fused indane sub-units, sharing the common apical carbon of the five-membered rings. This provides a test of this assumption as the crystal structure is known.41 It consists of stacks of molecules aligned along the c-axis, with the closest intermolecular distance being H⋯H contacts at 2.220 Å. Fig. 5 compares the experimental INS spectrum with those calculated using the isolated molecule approximation (Gaussian) and the full periodic calculation (CASTEP). It can be seen that both methods are in good agreement with the data and while the CASTEP calculation produces a better overall match, the differences are small, justifying the use of isolated molecule calculations.For molecular solids such as these it might be expected that inclusion of long-range interactions would be required to obtain good agreement between experiment and theory. We have investigated this possibility for both intermolecular and intramolecular consequences. As explained further in the ESI,† the effect of the inclusion of the long-range interactions via the Tkatchenko and Scheffler36 (TS) correction is very small for the systems studied here.
Fig. S8–S12 (ESI†) compare the calculated and observed INS and infrared spectra for the centropolyindanes 2–6. In all cases the agreement is acceptable and enables assignments to be made. As suggested by the comparison with indane, the strong infrared features at 450, 750 and 1480 cm−1 are assigned as phenylene modes (the Wilson52 labelling of the parent benzene modes is given in brackets): out-of-plane ring deformation (16b), C–H out-of-plane bend (11) and C–C stretch (19a), respectively. Similarly, for the Raman spectra, the strong mode at ∼1020 cm−1 is the phenylene C–H in-plane bend (18b). All of the centropolyindanes have a very strong Raman mode (note the difference in ordinate scales between the left and right sides of Fig. 3) at ∼100 cm−1, and compounds 2, 4, 5 and 6, but neither indane (1) nor tribenzotriquinacene (3) have a strong mode at ∼150 cm−1. (The same modes are also seen in the INS spectra.) Visualisation of the modes shows that the 100 cm−1 mode is related to the indane mode at ∼160 cm−1, (C9–C1 + C4–C3 in-phase torsion), that has shifted down in energy because of the replacement of hydrogen atoms by the heavier indane moieties. This fragment is common to all the molecules and thus appears in all their spectra. In contrast, the ∼150 cm−1 mode involves the central neopentane core and is formally derived from one component of ν19, a triply degenerate C–C–C bending motion.54 This explains why it is only seen in compounds 2, 4, 5 and 6: only these have the fully developed neopentane core. The modes are shown in Fig. 6 for fenestrindane (6), together with a comparison of the calculated spectra for the isolated molecule (Z = 1) and the primitive cell (Z = 2). It can be seen that for these very low energy modes there is a small, but significant, influence of the intermolecular interactions.
At the opposite end of the spectra, the C–H stretch region is remarkably simple (Fig. 7). In the aromatic region, >3000 cm−1, for both the Raman and the infrared spectra the pattern found for indane is largely replicated, consisting of two and three bands, respectively. This indicates that, in all the samples, the phenylene groups of the indane residues behave as independent entities, there is little or no coupling between them. The aliphatic region shows more variety, but this is understandable because of the different types of C–H groups present. Thus indane (1) has three methylene groups, which, as the spectra indicate, are coupled (since there are more than the two modes expected for isolated oscillators: the symmetric, ∼2850 cm−1, and asymmetric, ∼2940 cm−1, H–C–H stretch modes). Triptindane (2) also has three methylene groups, but these are separated from each other by the central carbon atom. Nonetheless, the spectrum resembles that of indane. Compounds 3, 5 and 6 each contain four bridgehead methine groups but only show a single band at ∼2890 cm−1, in both the Raman and infrared spectra. Difuso-centrotriindane 4 bears two methine groups and two methylene groups; thus the spectrum shows the methylene doublet at 2840 and 2930 cm−1, and the methine band at 2900 cm−1.
The C–C stretch modes
Potentially, the most interesting modes are those that involve carbon atoms that are common to two or more indane sub-units because these reflect the strength of the bonding between the indane sub-units. However, as we have already seen, the spectra are dominated by the ortho-phenylene modes and the aliphatic framework does not contribute strong modes (except for the low energy deformation mode described previously). The DFT-calculated INS spectra provide a means to access the aliphatic C–C stretch modes. By setting the cross sections of all the atoms to zero except those of interest, the resulting “pseudo-spectra” only contain modes where the atom(s) of interest have significant motion. Fig. 8 shows the results of this exercise where only the carbon atom that is common to the most indane sub-units has a non-zero cross section. Thus for compounds 2, 3, 4 and 5 the common carbon atom links three indane sub-units and for compound 6 it links four indane sub-units. For tribenzotriquinacene (3) this is a tertiary carbon atom, for the hydrocarbons 2, 4, 5 and 6 it is a quaternary carbon atom.From Fig. 8, it can be seen that there are three regions where there is significant motion of the atom of interest: 450–650 cm−1, 1000–1150 cm−1 and 1250–1400 cm−1. Note that the pseudo-spectra only report modes where there is motion of the atom, not whether it is the driving force of the mode. Visualisation of the modes shows that the intensity in the 1250–1400 cm−1 region is a consequence of the requirement for a normal mode that the centre of mass is invariant, thus atoms can move simply to satisfy this criterion, even though there is no change in bond lengths or angles that involve the atoms. A similar situation is found for the modes at 450–650 cm−1, although in the case of compounds 2 and 3 the mode around 550 cm−1 does include contributions from C–C–C bending modes involving the atom of interest. The modes at 1000–1150 cm−1 do have large components of motion that contain C–C stretch modes of the central carbon atom. In triptindane (2), the C–C stretch of the central carbon atoms (the two common to all three indane sub-units) is calculated at 1047 cm−1 (1036 cm−1 observed). The similarity of the transition energy in all cases indicates that the bonding is also of similar strength; the additional indane unit makes little difference to the transition energy for fenestrindane (6). What is apparent is that the local symmetry around the carbon atom is important: for compounds 2 and 3 it is trigonal (C3v) and this gives two modes, for compounds 4, 5 and 6 it is tetrahedral (Td). This results in a triply degenerate asymmetric stretch mode (T2) and a non-degenerate symmetric (A1) C–C stretch mode. While the local symmetry is Td, the molecular symmetry is lower and this lifts the degeneracy of the T2 mode, so for 6, the mode at 1065 cm−1 actually consists of three components, calculated at 1063, 1069 and 1070 cm−1. For 4, 5 and 6 the A1 mode is found at 671, 661 and 657 cm−1 respectively. This is either very weak or absent from the spectra in Fig. 8 because in Td symmetry the mode does not involve displacement of the central atom. For comparison, in neopentane54 the C–C stretch modes occur at 924 (T2) and 733 cm−1 (A1).
The effect of substitution
The compounds investigated also enable insights into how substitution of the indane framework (2, 7 and 8) and substitution at the central carbons (3, 9 and 10) modifies the spectra.The carbonyl peak position in 7 is unusual: in cyclopentanone it occurs at 1746 cm−1, well above the usual range of 1710–1720 cm−1 for dialkyl ketones; the difference is ascribed to the effects of ring strain.53 More generally, alkyl–aryl ketones show C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch bands in the range 1680–1690 cm−1.55 Thus aryl substitution decreases the carbonyl transition energy by ∼30 cm−1, which is consistent with the carbonyl transition energy of 1-indanone being 1715 cm−1. The further small decrease must be the result of a long-range interaction with the two other indane rings, since in benzophenone the carbonyl transition energy is 1660 cm−1. That this is an electronic effect and not a ring strain effect is demonstrated by the C–C(
O stretch bands in the range 1680–1690 cm−1.55 Thus aryl substitution decreases the carbonyl transition energy by ∼30 cm−1, which is consistent with the carbonyl transition energy of 1-indanone being 1715 cm−1. The further small decrease must be the result of a long-range interaction with the two other indane rings, since in benzophenone the carbonyl transition energy is 1660 cm−1. That this is an electronic effect and not a ring strain effect is demonstrated by the C–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–C angles in cyclopentanone,56 1-indanone57 and triptindan-9-one (7) being 108.3/109.1° (there are two independent molecules in the unit cell), 107.5° and 107.3° (calculated) respectively, i.e. essentially the same, but showing a 40 cm−1 difference in transition energy.
O)–C angles in cyclopentanone,56 1-indanone57 and triptindan-9-one (7) being 108.3/109.1° (there are two independent molecules in the unit cell), 107.5° and 107.3° (calculated) respectively, i.e. essentially the same, but showing a 40 cm−1 difference in transition energy.
The new peaks at 1077, 1089, 1251 cm−1 and the increased width of the modes at ∼1450 cm−1 in 1,4-dimethoxytriptindane (8) are all associated with the aryl–O–CH3 functionality. They are assigned as: the O–CH3 stretches (1077, 1089 cm−1), the aryl–O stretch (1251 cm−1) and the symmetric and asymmetric methyl deformations (1435–1470 cm−1).55
The C–H stretch region of ketone 7 is very similar to that of the parent hydrocarbon 2 apart from a general weakening of the bands. For the dimethoxy compound 8, this region is made more complex by the addition of the symmetric and asymmetric methyl C–H stretch modes at the usual positions (∼2880 and 2980 cm−1).55
It might be expected that the addition of more methyl groups in the fully methylated tribenzotriquinacene 10 would simply result in a corresponding number of very intense torsional modes. This is not the case; rather, a complex manifold of modes is observed (Fig. 10). The DFT calculations show that the four methyl torsions occur at: 239 (265), 277 (293) and 392 (387) cm−1 (experimental values in brackets). Visualisation of the modes provides two surprises: firstly, although the 293 cm−1 mode in the tetramethyl derivative 10 is at the same transition energy as for the monomethyl analogue 9, it does not involve the central methyl group, only the peripheral ones. It is a doubly degenerate mode, so its intensity is doubled, otherwise all of the modes would have comparable intensity. Secondly, the central methyl's torsion occurs at 387 cm−1 and, in contrast to the other torsional modes (which are almost pure torsions), it is highly mixed with the tribenzotriquinacene framework modes. A methyl torsion at this energy is completely unprecedented.
In the C–H stretch region, the aromatic modes above 3000 cm−1 are very similar for all three tribenzotriquinacenes 3, 9 and 10. For the parent material, 3, with four methine C–H groups, the classical methine stretch at 2901 cm−1 is seen, involving all four methines. In addition there is a band at 2976 cm−1 which is almost a pure C–H stretch of the central methine group. On substitution by the methyl group in hydrocarbon 9, this vanishes and is replaced by weak modes at 2863 cm−1 (CH3 symmetric stretch) and 2924/2959 cm−1 (CH3 asymmetric stretch). Complete substitution of the methine hydrogens in compound 10 by methyl groups results in the disappearance of the 2901 cm−1 band and the appearance of a cluster of bands in the regions expected for the symmetric and asymmetric methyl stretch modes.55
Conclusions
The vibrational spectra of a set of various centropolyindanes has provided insights into the bonding present and some surprises. The spectra clearly show that the ortho-phenylene rings of the indane sub-units largely behave as independent entities. There is no evidence for any significant coupling between them, as demonstrated by the similarity of the spectra, both to each other and to the parent molecule, indane (1). The C–C stretch modes of the carbon atoms that are common to two or more indane sub-units occur in the usual range associated with sp3 C–C bonds, suggesting that these are conventional bonds.The surprises occur on substitution of the molecules. Thus the carbonyl stretch mode of triptindan-9-one (7) is lower than would be expected. This is not a ring strain effect, but appears to be a longer-range effect of the additional ortho-phenylene rings. Methyl substitution in the tribenzotriquinacene derivatives 9 and 10 produces methyl torsional modes that are the highest known. The assignments provided here are a reference for future studies of these unusual hydrocarbons.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The STFC Rutherford Appleton Laboratory is thanked for access to neutron beam facilities. Computing resources (time on the SCARF compute cluster for the CASTEP and Gaussian calculations) was provided by STFC's e-Science facility. This research has been performed with the aid of facilities at the Research Complex at Harwell (RCaH), including the FT-Raman spectrometer. The authors would like to thank the Research Complex for access and support to these facilities and equipment.Notes and references
- H. Hopf, Classics in Hydrocarbon Chemistry, Wiley-VCH, Weinheim, 2000 Search PubMed.
- A. Narita, X.-Y. Wang, X. Feng and K. Müllen, Chem. Soc. Rev., 2015, 44, 6616–6643 RSC.
- E. L. Spitler, C. A. Johnson II and M. M. Haley, Chem. Rev., 2006, 106, 5344–5386 CrossRef CAS PubMed.
- V. M. Tsefrikas and L. T. Scott, Chem. Rev., 2006, 106, 4868–4884 CrossRef CAS PubMed.
- T.-Y. Wu and J. S. Siegel, Chem. Rev., 2006, 106, 4843–4867 CrossRef PubMed.
- Y. Segawa, A. Yagi, K. Matsui and K. Itami, Angew. Chem., 2016, 128, 5222–5245 ( Angew. Chem., Int. Ed. , 2016 , 55 , 5136–5158 ) CrossRef.
- K. Y. Cheung, X. Xu and Q. Miao, J. Am. Chem. Soc., 2015, 137, 3910–3914 CrossRef CAS PubMed.
- J.-W. Han, X. Li and H. N. C. Wong, Chem. Rec., 2015, 15, 107–131 CrossRef CAS PubMed.
- K. Ikemoto, J. Lin, R. Kobayashi, S. Sato and H. Isobe, Angew. Chem., 2018, 130, 8691–8695 ( Angew. Chem., Int. Ed. , 2018 , 57 , 8555–8559 ) CrossRef.
- Fragments of Fullerenes and Carbon Nanotubes, ed. M. A. Petrukhina and L. T. Scott, Wiley, Hoboken, 2012 Search PubMed.
- C. Thilgen and F. Diederich, Chem. Rev., 2006, 106, 5049–5135 CrossRef CAS PubMed.
- D. Wendinger and R. R. Tykwinski, Acc. Chem. Res., 2017, 50, 1468–1479 CrossRef CAS PubMed.
- P. Gund and T. M. Gund, J. Am. Chem. Soc., 1981, 103, 4458–4465 CrossRef CAS.
- Diamond and Related Nanostructures, Vol. 6 of Carbon Materials: Chemistry and Physics (F. Cataldo and P. Milani, Series Eds.), ed. M. V. Diudea and C. L. Nagy, Springer, Dordrecht, 2013 Search PubMed.
- M. Mastalerz, Acc. Chem. Res., 2018, 51, 2411–2422 CrossRef CAS PubMed.
- F. Beuerle and B. Gole, Angew. Chem., 2018, 130, 4942–4972 ( Angew. Chem., Int. Ed. , 2018 , 57 , 4850–4878 ) CrossRef.
- X. Shen, D. M. Ho and R. A. Pascal Jr., Org. Lett., 2003, 5, 369–371 CrossRef CAS PubMed.
- D. Kuck, Chem. Rev., 2006, 106, 4885–4925 CrossRef CAS PubMed.
- H. W. Thompson, J. Org. Chem., 1968, 33, 621–625 CrossRef CAS.
- B. Paisdor and D. Kuck, J. Org. Chem., 1991, 56, 4753–4759 CrossRef CAS.
- D. Kuck, Angew. Chem., 1984, 23, 508–509 ( Angew. Chem., Int. Ed. Engl. , 1984 , 96 , 515–516 ) Search PubMed.
- D. Kuck, T. Lindenthal and A. Schuster, Chem. Ber., 1992, 125, 1449–1460 CrossRef CAS.
- G. Markopoulos, L. Henneicke, J. Shen, Y. Okamoto, P. G. Jones and H. Hopf, Angew. Chem., 2012, 124, 13057–13060 ( Angew. Chem., Int. Ed. , 2012 , 51 , 12884 ) CrossRef.
- D. Kuck and H. Bögge, J. Am. Chem. Soc., 1986, 108, 8107–8109 CrossRef CAS.
- D. Kuck and A. Schuster, Angew. Chem., 1988, 100, 1222–1224 ( Angew. Chem., Int. Ed. Engl. , 1988 , 27 , 1192–1194 ) CrossRef.
- H.-W. Ip, C.-F. Ng, H.-F. Chow and D. Kuck, J. Am. Chem. Soc., 2016, 138, 13778–13781 CrossRef CAS PubMed.
- W.-S. Wong, C.-F. Ng, D. Kuck and H.-F. Chow, Angew. Chem., 2017, 129, 12528–12532 ( Angew. Chem., Int. Ed. , 2017 , 56 , 12356–12360 ) CrossRef.
- For example, see: D. Kuck, J. Linke, L. C. Teichmann, D. Barth, J. Tellenbröker, D. Gestmann, B. Neumann, H.-G. Stammler and H. Bögge, Phys. Chem. Chem. Phys., 2016, 18, 11722–11737 RSC.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, B72, 171–179 CrossRef PubMed.
- D. Kuck, A. Schuster, R. A. Krause, J. Tellenbröker, C. P. Exner, M. Penk, H. Bögge and A. Müller, Tetrahedron, 2001, 57, 3587–3613 CrossRef CAS.
- S. F. Parker, F. Fernandez-Alonso, A. J. Ramirez-Cuesta, J. Tomkinson, S. Rudić, R. S. Pinna, G. Gorini and J. Fernández Castañon, J. Phys.: Conf. Ser., 2014, 554, 012003 CrossRef.
- M. A. Adams, S. F. Parker, F. Fernandez-Alonso, D. J. Cutler, C. Hodges and A. King, Appl. Spectrosc., 2009, 63, 727–732 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 09, Revision D.02, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson and M. C. Payne, Z. Kristallogr., 2005, 220, 567 CAS.
- K. Refson, P. R. Tulip and S. J. Clark, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 155114 CrossRef.
- A. Tkatchenko and M. Scheffler, Phys. Rev. Lett., 2009, 102, 073005 CrossRef PubMed.
- R. Dennington, T. A. Keith and J. M. Millam, GaussView, Version 5.0.9, Semichem Inc., Shawnee Mission, KS, 2016 Search PubMed.
- A. J. Ramirez-Cuesta, Comput. Phys. Commun., 2004, 157, 226–238 CrossRef CAS.
- N. M. O'Boyle, A. L. Tenderholt and K. M. Langner, J. Comput. Chem., 2008, 29, 839–845 CrossRef PubMed.
- M. Harig, B. Neumann, H.-G. Stammler and D. Kuck, ChemPlusChem, 2017, 82, 1078–1095 CrossRef CAS , see ref. 76 cited therein.
- J. G. Brandenburg, S. Grimme, P. G. Jones, G. Markopoulos, H. Hopf, M. K. Cyranski and D. Kuck, Chem. – Eur. J., 2013, 19, 9930–9938 CrossRef CAS PubMed.
- M. I. Davis and T. W. Muecke, J. Phys. Chem., 1970, 74, 1104–1108 CrossRef CAS.
- S. F. Parker, D. Siegel, N. G. Hamilton, J. Kapitán, L. Hecht and D. Lennon, J. Phys. Chem. A, 2012, 116, 333–346 CrossRef CAS PubMed.
- J. F. Fuller and E. J. Valente, J. Chem. Crystallogr., 1996, 26, 815–821 CrossRef CAS.
- Y. Li, L. Zhang, Z. Zhang, J. Xu, Y. Pan, C. Xu, L. Liu, Z. Li, Z. Yu, H. Li and L. Xu, Adv. Synth. Catal., 2016, 358, 2148–2155 CrossRef CAS.
- L. B. Favero, W. X. Li, G. Spadini and W. Caminati, J. Mol. Spectrosc., 2015, 316, 45–48 CrossRef CAS.
- A. P. Scott and L. Radom, J. Phys. Chem., 1996, 100, 16502–16513 CrossRef CAS.
- T. L. Smithson, J. A. Duckett and H. Wieser, J. Phys. Chem., 1984, 88, 1102–1109 CrossRef CAS.
- K. H. Hassan and J. M. Hollas, J. Mol. Spectrosc., 1991, 147, 100–113 CrossRef CAS.
- Z. Arp, N. Meinander, J. Choo and J. Laane, J. Chem. Phys., 2002, 116, 6648–6655 CrossRef CAS.
- L. Bardet, G. Fleury, R. Granger and C. Sablayrolles, J. Mol. Struct., 1968, 2, 397–408 CrossRef CAS.
- G. Varsanyi, Assignments for vibrational spectra of 700 benzene derivatives, Akadémiai Kiadó, Budapest, 1973 Search PubMed.
- F. R. Dollish, W. G. Fateley and F. F. Bentley, Characteristic Raman frequencies of organic compounds, John Wiley & Sons, New York, 1974 Search PubMed.
- H. Jobic, S. Sportouch and A. Renouprez, J. Mol. Spectrosc., 1983, 99, 47–55 CrossRef CAS.
- D. Lin-Vien, N. B. Colthup, W. G. Fateley and J. G. Grasselli, The handbook of infrared and Raman characteristic frequencies of organic molecules, Academic Press, Boston, 1991 Search PubMed.
- D. S. Yufit and J. A. K. Howard, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2011, 67, o104–o106 CrossRef CAS PubMed.
- T. P. Ruiz, M. Fernandez-Gomez, J. J. L. Gonzalez, A. E. Koziol and J. M. G. Roldan, J. Mol. Struct., 2004, 707, 33–46 CrossRef.
- D. A. Braden, S. F. Parker, J. Tomkinson and B. S. Hudson, J. Chem. Phys., 1999, 111, 429–437 CrossRef CAS.
- J. Tomkinson, S. F. Parker, D. A. Braden and B. S. Hudson, Phys. Chem. Chem. Phys., 2002, 4, 716–721 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of compound 8, structure and spectra of compounds 1–10, comparisons of observed and calculated infrared and INS spectra. See DOI: 10.1039/c8cp07311b |
| This journal is © the Owner Societies 2019 |