Production of HO2 and OH radicals from near-UV irradiated airborne TiO2 nanoparticles
Abstract
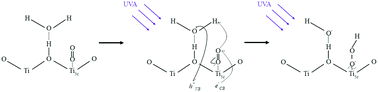
The production of gas-phase hydroperoxyl radicals, HO2, is observed directly from sub-micron airborne TiO2 nanoparticles irradiated by 300–400 nm radiation. The rate of HO2 production as a function of O2 pressure follows Langmuir isotherm behaviour suggesting O2 is involved in the production of HO2 following its adsorption onto the surface of the TiO2 aerosol. Reduction of adsorbed O2 by photogenerated electrons is likely to be the initial step followed by reaction with a proton produced via oxidation of adsorbed water with a photogenerated hole. The rate of HO2 production decreased significantly over the range of relative humidities between 8.7 and 36.9%, suggesting competitive adsorption of water vapour inhibits HO2 production. From the data, the adsorption equilibrium constants were calculated to be: KO2 = 0.27 ± 0.02 Pa−1 and KH2O = 2.16 ± 0.12 Pa−1 for RH = 8.7%, decreasing to KO2 = 0.18 ± 0.01 Pa−1 and KH2O = 1.33 ± 0.04 Pa−1 at RH = 22.1%. The increased coverage of H2O onto the TiO2 aerosol surface may inhibit HO2 production by decreasing the effective surface area of the TiO2 particle and lowering the binding energy of O2 on the aerosol surface, hence shortening its desorption lifetime. The maximum yield (i.e. when [O2] is projected to atmospherically relevant levels) for production of gas-phase HO2, normalised for surface area and light intensity, was found to be 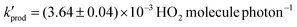 at a RH of 8.7% for the 80% anatase and 20% rutile formulation of TiO2 used here. This yield decreased to
at a RH of 8.7% for the 80% anatase and 20% rutile formulation of TiO2 used here. This yield decreased to 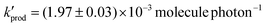 as the RH was increased to 22.1%. Using this value, the rate of production of HO2 from TiO2 surfaces under atmospheric conditions was estimated to be in the range 5 × 104–1 × 106 molecule cm−3 s−1 using observed surface areas of mineral dust at Cape Verde, and assuming a TiO2 fraction of 4.5%. For the largest loadings of dust in the troposphere, the rate of this novel heterogeneous production mechanism begins to approach that of HO2 production from the gas-phase reaction of OH with CO in unpolluted regions. The production of gas-phase OH radicals could only be observed conclusively at high aerosol surface areas, and was attributed to the decomposition of H2O2 at the surface by photogenerated electrons.
as the RH was increased to 22.1%. Using this value, the rate of production of HO2 from TiO2 surfaces under atmospheric conditions was estimated to be in the range 5 × 104–1 × 106 molecule cm−3 s−1 using observed surface areas of mineral dust at Cape Verde, and assuming a TiO2 fraction of 4.5%. For the largest loadings of dust in the troposphere, the rate of this novel heterogeneous production mechanism begins to approach that of HO2 production from the gas-phase reaction of OH with CO in unpolluted regions. The production of gas-phase OH radicals could only be observed conclusively at high aerosol surface areas, and was attributed to the decomposition of H2O2 at the surface by photogenerated electrons.
- This article is part of the themed collections: 2019 PCCP HOT Articles and 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...