Isotope-selective chemistry in the Be+(2S1/2) + HOD → BeOD+/BeOH+ + H/D reaction
Abstract
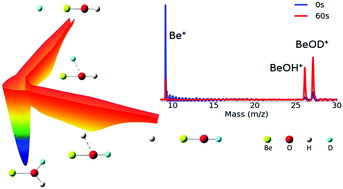
Low temperature reactions between laser-cooled Be+(2S1/2) ions and partially deuterated water (HOD) molecules have been investigated using an ion trap and interpreted with zero-point corrected quasi-classical trajectory calculations on a highly accurate global potential energy surface for the ground electronic state. Both product channels have been observed for the first time, and the branching to BeOD+ + H is found to be 0.58 ± 0.14. The experimental observation is reproduced by both quasi-classical trajectory and statistical calculations. Theoretical analyses reveal that the branching to the two product channels is largely due to the availability of open states in each channel.
- This article is part of the themed collections: 2019 PCCP HOT Articles, Photodissociation and reaction dynamics and 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
