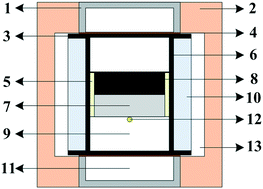
FeS is the main sulfur-containing compound in natural diamond inclusions. The research on FeS-doped diamond crystals plays an extremely important role in exploring the growth environment of natural diamond and the chemical composition of the earth. In our work, the characteristics of FeS doped diamond crystals were studied by using a China-type large volume cubic high-pressure apparatus with an iron–nickel alloy as a catalyst at 6.0 GPa and 1300–1350 °C. The crystals were characterized by OM, SEM, XPS, FTIR and Raman. Scanning electron microscopy of the crystal shows that the {100} plane of the crystal is rougher than {111}, which is related to the number of dangling bonds on different crystal faces of the crystal. The residue on the surface of the crystal was detected by mapping, and the test result showed that it was a sulfur iron and a sulfur nickel compound. FeS will decompose in the synthesis system, and the decomposition products are Fe and S2.The XPS spectrum of the crystal shows that S was successfully incorporated into the diamond lattice by forming C–S–O and C–S–SO2–C bonds, and the peak intensity was strong. The infrared spectrum shows that the N content in the crystal gradually increases as the doping ratio increases. Raman spectroscopy analysis shows that the S-doped Ib-type diamond single crystal has a high-quality sp3 structure, and the full width at half maximum (FWHM) of the crystal gradually broadens as the doping ratio increases.