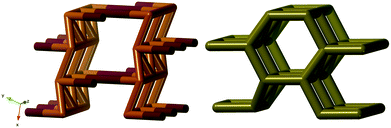Open Access Article
Open Access ArticleNatural and synthetic metal oxalates – a topology approach†
Cyrielle L. F.
Dazem
a,
Francoise M.
Amombo Noa
 b,
Justin
Nenwa
a and
Lars
Öhrström
b,
Justin
Nenwa
a and
Lars
Öhrström
 *b
*b
aInorganic Chemistry Department, Faculty of Science, University of Yaoundé I, PO. Box 812, Yaoundé, Cameroon. E-mail: cyrielle_leyla@yahoo.fr; jnenwa@yahoo.fr
bChemistry and Biochemistry, Dept. of Chemistry and Chemical Engineering, Chalmers University of Technology, SE-41296 Göteborg, Sweden. E-mail: mystere@chalmers.se; ohrstrom@chalmers.se
First published on 16th September 2019
Abstract
Oxalates are found in minerals and in biology, are made in the laboratory, and are used on an industrial scale. They form coordination polymers and hydrogen bonded networks that often can be analysed using network topology. In this survey of known naturally occurring oxalates we note weddellite, [Ca(C2O4)]·xH2O, that seems to be the first known naturally occurring metal–organic framework, forming the four-connected crb-net (zeolite CRB). The natural oxalates are typically 3D, 2D or 1D coordination polymers, with extensive hydrogen bonding in the latter cases. For example, humboldtine and lindbergite form the new 3- and 8-connected net hum by combining the 1D structure with strong hydrogen bonds. Tris-oxalates rarely occur in nature but stepanovite, [Mg(H2O)6][Na[Fe(ox)3]]·3H2O, is an exception and forms hcb-nets (honeycomb 2D layers) with the hexaaqua ions sealing any potential voids. Synthetic tris-oxalates on the other hand are well explored and normally form 2D hcb-nets or 3D chiral three-connected srs-nets. Theoretically a few other topologies should also be possible, and it was found that [Mn((R)-salmen)(CH3OH)(CH3CN)][MnCr(ox)3]·0.5CH3OH·1.25CH3CN forms the achiral three-connected lig-net, [Fe(2,6-bis(pyrazol-3-yl)pyridine)2][MnCr(ox)3]·2,6-bis(pyrazol-3-yl)pyridine·CH3OH forms the likewise achiral nod-net and [Cu(trans[14]dien)][KCr(ox)3] the ths-net. A new binodal 3-connected net noa (with the derived 3c-,4c- new net mys) was found in [FeII(tren(imid)3)]2[Mn2.5(CH3OH)3Cr3(ox)9]·(CH3OH)4.75·(H2O)4. The more complex [Fe(tren(imid)3)]2[Mn2.5(CH3OH)3Cr3(ox)9] forms a new three-nodal 3-connected daz-net.
1. Introduction
Oxalic acid and oxalates date far back in human history, both found as a tasty part of numerous plants, and in painful and dangerous kidney and bladder stones. In industry, they are used in the separation of various metals, and other practical examples employing the ubiquitous C2O42− unit are in artistic chemical photography processes and the anticancer drug oxaliplatin (trade name Eloxatin).1 But oxalates are also very much present in today's cutting edge science.2–7 Recently, for example, MOFs from tetrakis oxalates with zeolite topologies have been prepared.3Tri-potassium trioxalato-rhodium monohydrate was the focus of the first diffraction study of oxalates in 1927 (ZZZVZK)8 followed by beryllium oxalate trihydrate in 1928 (ZZZQZK), and today the Cambridge Crystallographic Data Centre has collected over 6000 oxalate entries into the CSD.
Due to the possibility of the oxalate ion forming bridging chelates in two or more directions, and its many hydrogen bond accepting sites, oxalate compounds often form networks. The topological analysis of such networks has its origins in the work of Alexander Wells starting in the 1950s,9,10 gaining popularity by the analysis of coordination polymers and metal–organic frameworks (MOFs) in the 1990s and onwards.11–13
Here, we report how well metal compounds with small oxalate ions (ox) lend themselves to such topological analysis. We will show the importance of network topologies in both the synthesis of new oxalate compounds and in the communication and understanding of known oxalates, both natural and synthetic.14
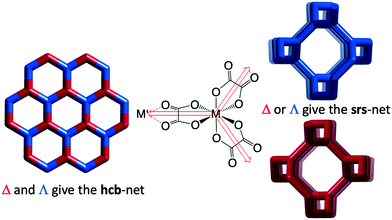
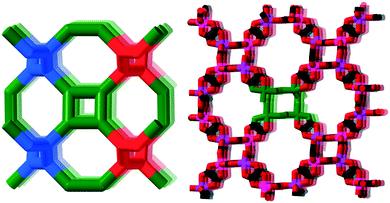
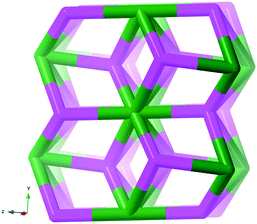
We have investigated two cases, first the tris-oxalates, [M′M(ox)3], forming three-connected networks. These are known to produce 2D hcb-nets (honeycomb nets, Fig. 1 left) or the 3D chiral srs-nets (or (10,3)-a, Fig. 1 right), but geometrical considerations suggest that also a number of other 3-connected topologies should be conceivable, even though the srs-net appears to be the perfect fit for enantiomerically pure tris-oxalates.13,15
We do not consider the four-connecting tetrakis-oxalates here if they are not naturally occurring, as these have been discussed elsewhere.3,16
Secondly, we consider the natural oxalates, recently reviewed by Piro and Baran.17 Through the 2D tris-oxalate hcb-nets there is some overlap with the first group,18 and the rare Siberian mineral stepanovite had its 15 minutes of fame a few years ago19,20 when the structures were, unexpectedly, found to resemble “well-established magnetic and proton-conducting metal oxalate MOFs”.21
2. Experimental
2.1 Materials and methods
3. Results and discussion
3.1 Tris-oxalates, [M′M(ox)3]x−
The Cambridge Structural Database in total contains 682 entries with a minimum of three bridging oxalates (15 added to the latest 5.40 tri-annual update), and at least 301 of these are tris-oxalate networks either with M = M′ or with different M and M′. A recent survey of srs-nets (Fig. 1) lists 91 tris-oxalates having the srs-network topology,26 and as not all of the remaining ones form the 2D hcb-net (Fig. 1), we wondered about these network topologies. Is there one particular topology that is the second “choice” or are there many possibilities? And are they all chiral as the srs-net?In Table 1, we have collected the tris-oxalates from the CSD that form neither the srs nor hcb-net.
| M′M | Cation | CSD | Net |
|---|---|---|---|
| a Severely disordered. b New topologies. | |||
| Mn(II) Cr(III) | [Mn((S)-salmen)(CH3OH)2]+ | BEWVEG | lig |
| Mn(II) Cr(III) | [Mn((R)-salmen)(CH3OH)2]+ | BEWVOQ | lig |
| Mn(II) Cr(III) | [Fe(imid2-trien)]2+ | VINHOR | lig |
| Mn(II) Cr(III) | [Fe(tren(6-Me-py)3)]2+ | VINHIL | lig |
| Mn(II) Cr(III) | [Fe(2,6-bis(pyrazol-3-yl)pyridine)2]2+a | EDATOT | nod |
| Mn(II) Cr(III) | [Fe(sal2-trien)]3+ | GURPIT | nod |
| Mn(II) Cr(III) | [In(sal2-trien)]3+ | GURPOZ | nod |
| Mn(II) Cr(III) | [In(sal2-trien)]3+ | GURPUF | nod |
| Mn(II) Cr(III) | [Fe(5-CH3Osal2-trien)]3+ | PUWZOX | nod |
| Mn(II) Cr(III) | [Mn(salen)(H2O)]22+ | PIQFIG | nod |
| Mn(II) Cr(III) | (H2PPD+)(benzo[18]crown-6)2 | RUGKEL | nod |
| K(I) Cr(III) | [Cu(trans[14]dien)]2+ | QIYXIF | ths |
| Cu(II) | HN(Et)2(CH2CH2OH)+ | KIRFIA | ths |
| Cu(II) | HN(Et)3+ | KEDJAG | ths |
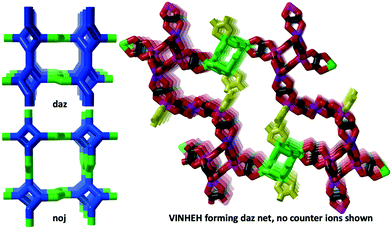
| Mn(II) Cr(III) | [Fe(tren(imid)3)]22+ | VINHEH | daz |
| Mn(II) Cr(III) | (o-FAni+)2(DCH[18]crown-6)2 | RUGKIP | noa |
Compared to the nets discussed by Öhrström & Larsson that were all formed from 10-gons as the shortest rings between nodes, the lig-net also forms 8-rings (point symbol 8210 and vertex symbol 8·8·103 (ref.15)). This and other three-connected nets are discussed in detail in ref. 13.
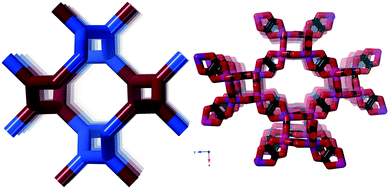
We found this net in for example [Cu(trans[14]dien)][KCr(ox)3],31 where trans[14]dien is 5,7,7,12,14,14-hexamethyl-1,4,8,11-tetraazacyclotetradeca-4,11-diene. In Fig. 4, it is shown (right) together with the ideal version of the ths-net (left).
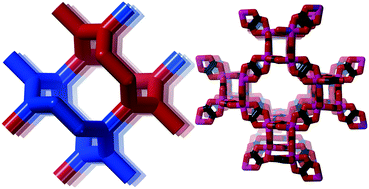
It has not escaped us that the noa-net is an augmented form of a three- and four-connected net with the four connected nodes in the centre of the squares. Somewhat surprisingly this is not the known jph-net16 but yet another new topology, mys, with point symbols 6·82 and 62·82·102. They differ again, just as lig and nod, in the arrangement of the four-fold helices of opposite chirality, see ESI† Fig. S1.
The resulting network topology is tri-nodal and chiral, but would be of limited interest to the crystal engineering community if it was not for its clear relationship to the nets already discussed and to other similar nets in the RCSR. The point symbols are 83, 83, and 8·102 and the Topos topological type is 3,3,3 T12.
As can be seen in Fig. 6 (left), the ideal daz-net resembles the srs-net, only with a zigzag motif added between helices along the z-axis. It is also related to the likewise chiral binodal noj-net that essentially is an srs-net with the zigzag motif inserted between all helices.
It is currently not known if there are similar versions of the achiral lig and nod nets.
We note, however, that the analysis of the structure using the daz-net greatly simplifies the task and renders “a very irregular 3D oxalate network”33 into a reasonably understandable geometrical object.
From this point of view it can be noted that the srs-net has a very good fit to the oxalate networks, (see ref. 13 and 15 for details) and counter ions like [M(1,10-bipyridine)3]n+ fit nicely inside. It is therefore not surprising that nets with similar densities like nod and lig form with cations of similar size.
BEWVEG and BEWVOQ are typical examples. The authors demonstrated that using the same cation, [Mn(R)-salmen]+ or [Mn(S)-salmen]+, the 3D oxalate network depends on the choice of solvent.28 When crystallized in acetonitrile, a 2D hcb-net resulted (BEWVIK and BEWVUW) instead of the 3D lig-net obtained from dichloromethane. Pertinent differences between the two structures are the configuration of the two phenoxy arms and the presence of different solvent molecules coordinated to MnIII in the two apical positions of the structure, [Mn(R/S)-salmen(CH3OH)2]+ for lig, whereas the hcb ones contain [Mn(R/S)-salmen(CH3CN)2]+ together with [Mn(R/S)-salmen(CH3OH)2]+.
VINHOR and VINHIL33 on the other hand, both lig-nets, get their network from the heptacoordinated MnII ions in each structure. The difference between these structures is that in VINHOR all the Mn atoms are heptacoordinated, and in VINHIL the second crystallographically independent Mn atom is hexacoordinated with a strong trigonal distortion of its octahedral coordination, which give rise to the presence of O–Mn–O angles of 154.6° instead of 180° expected for an octahedral coordination. The methanol in these two complexes is attached to the trioxalate network. Another remark on these two compounds is that the smaller number of cations necessary (+2 vs. +1 in i.e. BEWVEG) to compensate the negative charge of the oxalate network produces a more irregular network with heptacoordinated MnII ions coordinated to solvent molecules, and a more open structure with pores filled with disordered solvent molecules.
The nod-net in EDATOT34 may be related to a solvent effect. There is a presence of many interstitial solvent molecules since only one Fe complex is present per Mn/Cr pair, and results in no extra anions needed to stabilize the complex. The lower occupancy of cations and anions inside the anionic network could be the main cause for losing the enantiopure character of these systems.
Combining [FeIII(5-Xsal2-trien)]+ (X = NO2 or CH3O) with the MnIICrIII oxalates resulted in two different networks.35 The cation [FeIII(5-NO2sal2-trien)] gave a 2D hcb-net (PUWZIZ) since the electron withdrawing group favors π⋯π stacking interactions between phenyl rings of neighboring cationic complexes. In contrast, in PUWZOX the phenyls are not face to face. This is explained by the presence of the methoxy groups that hinders the face to face orientation of the neighboring rings. The dihedral angles, α, between the least-squares planes of the two phenoxy rings of the hcb-net are α = 89.002° ([Fe1] complex) and α = 94.248° ([Fe2] complex) and the dihedral angles, α, between the phenoxy rings in PUWZOX forming the nod-net are for [Fe1A], [Fe1B] and [Fe2] 110.47, 100.41 and 105.55°, respectively. The dihedral angles, α, between the least-squares planes of the two phenoxy rings have very different values in the 2D or 3D structures. Thus, the two phenoxy rings in the 2D compound (PUWZIZ) are closer to 90°, the ideal value for an octahedral complex.
3.2 Naturally occurring metal oxalates
Metal oxalates from minerals come in a large variety, from the class of tris-oxalates we have seen in the preceding section, to more or less discrete molecular entities held together by hydrogen bonds. While the former are normally straightforward to analyse, hydrogen bonded systems may be more ambiguous, and even simple metal oxalates may use multiple bonding sites on the oxalates, making some of these systems equally challenging.36In Table 3, we have assembled naturally occurring oxalates according to Piro and Baran17 with the network analysis performed by our group. We have mainly concentrated on the 3D and 2D coordination polymers, but the very simple humboldtine and lindbergite, the Fe and Mn versions of two isostructural 1D [M(C2O4)(H2O)2] oxalates, have also been included. In this latter case, the coordination network is supplemented with strong hydrogen bonds between coordinated water and oxalate ions.
| Compound | Formula | Net | Nodea |
|---|---|---|---|
| a ox = oxalate centroid. b New topology. | |||
| 3D coordination networks | |||
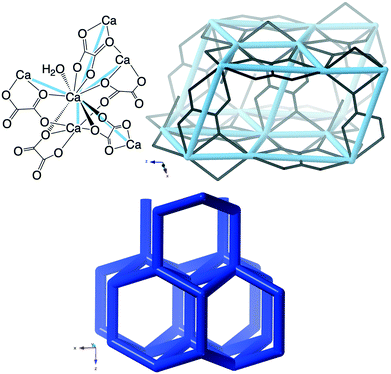
| Weddellite | [Ca(C2O4)(H2O)2]·xH2O | crb | Ca |
| Natroxalate | [Na2(C2O4)] | fit | Na, ox |
| Wheatleyite | [Na2[Cu(C2O4)2](H2O)2] | sxd | Na |
| Whewellite | [Ca(C2O4)H2O] | sqp | Ca |
| Novgorodavaite | [Ca2Cl2(C2O4)(H2O)2] | sqp | Ca |
| 2D coordination networks | |||
| Zhemchuzhnikovite | [Mg(H2O)6][AlNa(C2O4)3]·2H2O | hcb | Mg, Al |
| Stepanovite | [Mg(H2O)6][FeNa(C2O4)3]·3H2O | hcb | Mg, Fe |
| Deveroite | [Ce2(C2O4)3(H2O)6]·4H2O | hcb | Ce |
| Coskrenite | [Ce2(C2O4)(SO4)2(H2O)8] | hxl | Ce |
| 1D coordination networks or polymers | |||
| Humboldtine | [Fe(C2O4)(H2O)2] | hum | Fe, H2O |
| Lindbergite | [Mn(C2O4)(H2O)2] | hum | Mn, H2O |
| Levinsonite | [Al(H2O)6][Y(C2O4)(SO4)2(H2O)3]·3H2O | — | |
| Zugshunstite | [Al(H2O)6][Ce(C2O4)(SO4)2(H2O)3]·3H2O | — | |
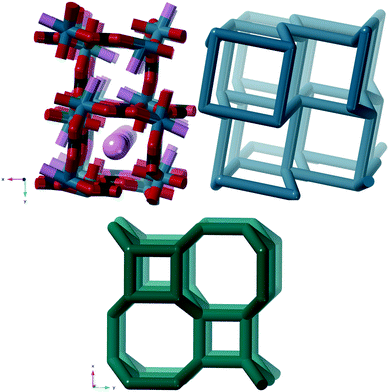
The crystal structure according to Tazzoli and Domeneghetti38 (CSD code WHWLTB) is shown in Fig. 8 together with the crb-net formed.
The porosity of weddellite has not been rigorously proven. However, in a study using an environmental scanning electron microscope with a heating stage attached, no changes in the crystal morphology were observed as water was desorbed37 and recently detailed variable temperature single crystal and powder X-ray diffraction (XRD) has confirmed structural integrity up to 105–140 °C after which the structure collapses.39
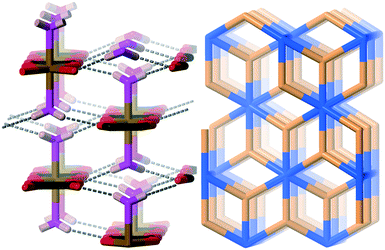
This forms the six-connected sxd-net and the network in the wheatleyite structure is shown in Fig. 10 together with the most symmetric form of the net.
 | ||
| Fig. 11 Top left: Whewellite, [Ca(C2O4)(H2O)], with every Ca2+ five-connected node in an sqp-net. Top right: The sqp-net in whewellite. Below: The ideal sqp-net. | ||
Inspecting this structure, one finds apparent voids, but they are all too small to be chemically significant.
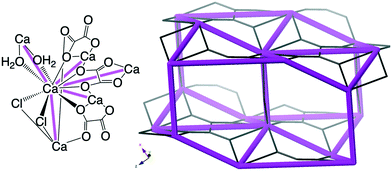 | ||
| Fig. 12 Top left: Novgorodavaite, [Ca2Cl2(C2O4)(H2O)2], with every Ca2+ five-connected node in an sqp-net. Top right: The sqp-net in novgorodavaite. The ideal sqp-net is shown in Fig. 11. | ||
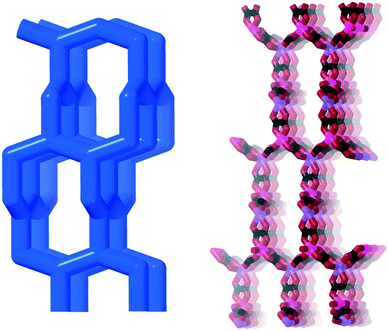
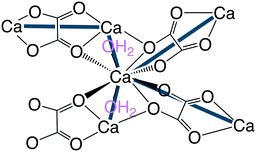
They have infinite bis-chelating –M–oxalate–M– chains with perpendicular coordinated water molecules, each hydrogen bonding to two oxalates. Taking the metal ions as one node and the water molecules as another, these structures form 3- and 8-connected nets. The water nodes are three-connected as they bind to the metal ion and then hydrogen bonds to two oxalates each. The metal ion node is eight connected as it binds to metal ions through coordination, two water molecules also via coordination, and then four waters via hydrogen bonds. This new topology hum is shown in Fig. 13 (right) together with the structure of humboldtine.
4. Conclusions
Analysis of the CSD data indicates that synthetic 3D tris-oxalates occur in more than one network topology, even if the srs-net is by far the most common. The well-known nets nod, lig, and ths were found to occur, together with the new topologies noa and daz. It also suggests that, as different topologies have differently shaped and sized cavities, a rational approach to design compounds with other network topologies not encountered so far, should be possible.Even so, the examples provided here give no clear guidance as how to perform such a rational design. Computational studies are in progress to address this question.
We also note that the issue may be more complex than a simple consideration of ideal geometries may suggest. Topologies stay the same even if bent, squeezed or otherwise distorted, as long as no bonds in the network are broken. Thus both the relative size and the shape of the empty space inside a particular network may be changed without changing the topology. Excellent examples are the “breathing” MOFs such as MIL-53.40 Indeed we suggest that it should be possible to calculate a figure of merit for each network topology on the relative energetic cost of different distortions to further enhance their utility as blueprints for the design of molecule based materials.
We also note that the synthetic tris-oxalates are often multi component systems where the network can interplay with one or more guests.41 Taking the network topology into consideration will be one important step in making the synthesis of such materials a more rational process.
The natural oxalates present a more complex picture, and in Fig. 14 we have tried to summarize and contrast them to the more symmetric synthetic analogues. The new net hum was described as a model for the bonding and structure in humboldtine and lindbergite.
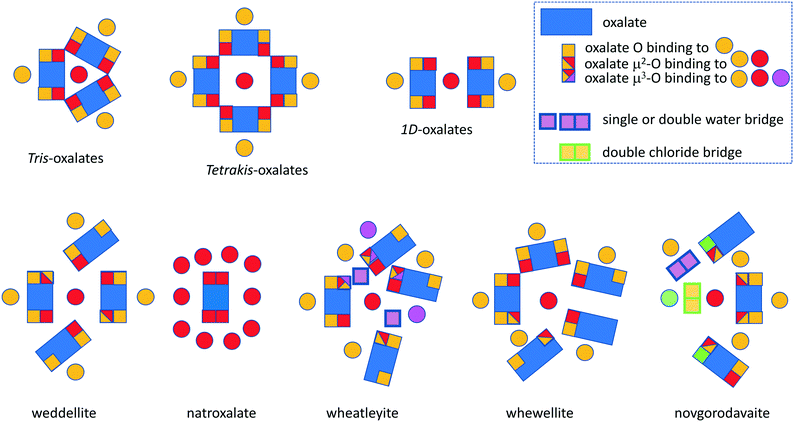 | ||
| Fig. 14 Schematic overview of the connectivity of the oxalates discussed in this article, including the tetrakis-oxalates only briefly mentioned. | ||
Conflicts of interest
There are no conflicts to declare.Acknowledgements
CLFD, FMAN, and LÖ thank the Swedish Research Council. We are grateful to Prof. Michael O'Keeffe for adding the new network topologies noa, mys, daz and hum to the RCSR database.Notes and references
- L. Öhrström and J. Covès, The Rhubarb Connection and Other Revelations: The Everyday World of Metal Ions, Royal Society of Chemistry, London, 2018 Search PubMed.
- I. Huskić, N. Novendra, D.-W. Lim, F. Topić, H. M. Titi, I. V. Pekov, S. V. Krivovichev, A. Navrotsky, H. Kitagawa and T. Friščić, Chem. Sci., 2019, 10, 4923–4929 RSC.
- F. Y. Yi, H. J. Yang, X. Zhao, P. Y. Feng and X. H. Bu, Angew. Chem., Int. Ed., 2019, 58, 2889–2892 CrossRef CAS PubMed.
- B. Zhang, P. J. Baker, Y. Zhang, D. Wang, Z. Wang, S. Su, D. Zhu and F. L. Pratt, J. Am. Chem. Soc., 2018, 140, 122–125 CrossRef CAS PubMed.
- M. Darawsheh, L. A. Barrios, O. Roubeau, S. J. Teat and G. Aromí, Angew. Chem., Int. Ed., 2018, 57, 13509–13513 CrossRef CAS PubMed.
- P. K. Tsobnang, E. Hastürk, D. Fröhlich, E. Wenger, P. Durand, J. L. Ngolui, C. Lecomte and C. Janiak, Cryst. Growth Des., 2019, 19, 2869–2880 CrossRef CAS.
- C. F. N. Nguemdzi, F. Capet, J. Ngouné, G. Bebga, M. Foulon and J. Nenwa, J. Coord. Chem., 2018, 71, 1484–1496 CrossRef CAS.
- G. W. Burgers and W. Bragg, Proc. R. Soc. London, Ser. A, 1927, 116, 553–586 CrossRef.
- A. F. Wells, Three-dimensional nets and polyhedra, John Wiley & Sons, New York, 1977 Search PubMed.
- A. F. Wells, Acta Cryst., 1954, 7, 535–544 CrossRef CAS.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed.
- V. A. Blatov, L. Carlucci, G. Giani and D. M. Proserpio, CrystEngComm, 2004, 6, 377–395 RSC.
- L. Öhrström and K. Larsson, Molecule-Based Materials The Structural Network Approach, Elsevier, Amsterdam, 2005 Search PubMed.
- L. Öhrström, Chem. – Eur. J., 2016, 22, 13758–13763 CrossRef PubMed.
- L. Öhrström and K. Larsson, Dalton Trans., 2004, 347–353 RSC.
- L. Öhrström and K. Larsson, Molecule-Based Materials: The Structural Network Approach, Elsevier, Amsterdam, 2005, ch. 12 Search PubMed.
- O. E. Piro and E. J. Baran, Crystallogr. Rev., 2018, 24, 149–175 CrossRef CAS.
- I. Huskic and T. Friscic, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2018, 74, 539–559 CrossRef CAS.
- B. Smith, 'Designer' materials found deep in Siberian permafrost, Cosmos, 2016, 08 AUGUST Search PubMed.
- E. Stoye, Naturally occurring MOFs found in coal mine minerals, Chemistry World, RSC, 2016, 19 AUGUST Search PubMed.
- I. Huskic, I. V. Pekov, S. V. Krivovichev and T. Friscic, Sci. Adv., 2016, 2, 7 Search PubMed.
- V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576–3586 CrossRef CAS.
- O. Delgado-Friedrichs and M. O'Keeffe, Acta Crystallogr., Sect. A: Found. Crystallogr., 2003, 59, 351–360 CrossRef PubMed.
- M. O'Keeffe, M. A. Peskov, S. Ramsden and O. M. Yaghi, Acc. Chem. Res., 2008, 41, 1782–1789 CrossRef PubMed.
- S. R. Batten, N. R. Champness, X. M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O'Keeffe, M. P. Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715–1724 CAS.
- A. Mizuno, Y. Shuku and K. Awaga, Bull. Chem. Soc. Jpn., 2019, 92, 1068–1093 CrossRef CAS.
- N. L. Rosi, J. Kim, M. Eddaoudi, B. Chen, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 1504–1518 CrossRef CAS PubMed.
- M. Clemente-León, E. Coronado and M. López-Jordà, Dalton Trans., 2013, 42, 5100–5110 RSC.
- V. A. Blatov, M. O'Keeffe and D. M. Proserpio, CrystEngComm, 2010, 12, 44–48 RSC.
- M. Clemente-León, E. Coronado, M. López-Jordà, G. Mínguez Espallargas, A. Soriano-Portillo and J. C. Waerenborgh, Chem. – Eur. J., 2010, 16, 2207–2219 CrossRef PubMed.
- H.-Y. Shen, W.-M. Bu, D.-Z. Liao, Z.-H. Jiang, S.-P. Yan and G.-L. Wang, Inorg. Chem., 2000, 39, 2239–2242 CrossRef CAS PubMed.
- T. Endo, K. Kubo, M. Yoshitake, S.-i. Noro, N. Hoshino, T. Akutagawa and T. Nakamura, Cryst. Growth Des., 2015, 15, 1186–1193 CrossRef CAS.
- A. Ben Djamâa, M. Clemente-León, E. Coronado and M. López-Jordà, Polyhedron, 2013, 64, 142–150 CrossRef.
- E. Coronado, J. R. Galán Mascarós, M. C. Giménez-López, M. Almeida and J. C. Waerenborgh, Polyhedron, 2007, 26, 1838–1844 CrossRef CAS.
- M. Clemente-León, E. Coronado and M. López-Jordà, Dalton Trans., 2010, 39, 4903–4910 RSC.
- C. Borel, M. Ghazzali, V. Langer and L. Öhrström, Inorg. Chem. Commun., 2009, 12, 105–108 CrossRef CAS.
- J. T. Kloprogge, T. E. Bostrom and M. L. Weier, Am. Mineral., 2004, 89, 245–248 CrossRef CAS.
- V. Tazzoli and C. Domeneghetti, Am. Mineral., 1980, 65, 327–334 CAS.
- A. R. Izatulina, V. V. Gurzhiy, M. G. Krzhizhanovskaya, M. A. Kuz'mina, M. Leoni and O. V. Frank-Kamenetskaya, Cryst. Growth Des., 2018, 18, 5465–5478 CrossRef CAS.
- L. R. Parent, C. H. Pham, J. P. Patterson, M. S. Denny, S. M. Cohen, N. C. Gianneschi and F. Paesani, J. Am. Chem. Soc., 2017, 139, 13973–13976 CrossRef CAS PubMed.
- M. Ghazzali, V. Langer, K. Larsson and L. Öhrström, CrystEngComm, 2011, 13, 5813–5817 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ce01187k |
| This journal is © The Royal Society of Chemistry 2019 |