Direction-specific fluorescence of an engineered organic crystal and the appearance of a new face caused by mechanically induced shaping†
Abstract
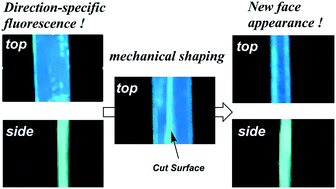
Here we have studied the directional nature of the fluorescence emission of a centimetre-scale organic crystal. Sky blue-colored fluorescence (λPL = 481 nm) of the (010) face and the green-colored fluorescence (λPL = 502 nm) of the (001) face were observed, because the (010) and (001) faces of the crystal have different directional orientations of the molecules. Interestingly, the mechanically induced shaping of the centimetre-scaled single crystal caused a new green-colored fluorescent (001) face to appear in the cross section.


 Please wait while we load your content...
Please wait while we load your content...