On the capability of metal–halogen groups to participate in halogen bonds
Abstract
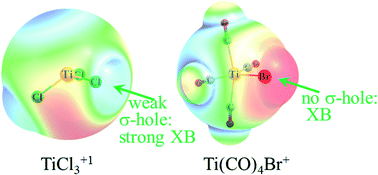
A number of halogen (X) atoms were covalently attached to a metal (M) and the ability of the X atom to act as electron acceptor in a halogen bond to nucleophile NCH was assessed. Both Cl and Br were considered as halogen atom, with NH3 and CO as other ligands attached to the metal. Metals tested were Ti, Mn, and Zn in various combinations of oxidation state, coordination, and overall charge. In the majority of cases, the strong electron-releasing power of the metal imbues the halogen atom with a high negative partial charge and minimizes the development of a σ-hole. As such, the M atom is generally a stronger attractor for the incoming nucleophile than is the halogen. Nonetheless, there are cases where a halogen bond can form such as Ti(CO)4Br+, TiCl3+, and MnCl4+, each with a different coordination. A requisite of halogen bond formation is generally an overall positive charge, although neutral species can engage in such bonds, albeit much weaker.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...