A unique copper coordination structure with both mono- and bi-dentate ethylenediamine ligands†
Abstract
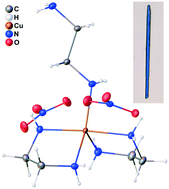
The compound (ethylenediamine-N)-bis(ethylenediamine-N,N′)-copper(II) bis(nitrate) is reported. It has an asymmetric unit containing Cu(II) penta-coordinating to two chelating ethylenediamine (en) and one monodentate en ligands with square-pyramidal-based geometry having severe trigonal distortion, together with two nitrate anions. It is the first crystallographic confirmation of the existence of penta-coordination with exclusively en ligands for any metal center, as well as the first monodentate en attached to Cu(II) seen in the solid state. It can be visualized as the penta-ammine effect realized solely with bi-amine ligands, defying the presumed necessity of mono-amines. X-ray crystallography establishes it to have P21/c space group with a = 12.83 Å, b = 9.77 Å, c = 11.91 Å, and β = 94.8° (at 100 K). Spectroscopic analysis of this compound is consistent with the crystallographic assessment of the coordination chemistry, and it is found to be a S = 1/2 paramagnet.


 Please wait while we load your content...
Please wait while we load your content...
