The role of the side chain in the conformational and self-assembly patterns of C2-symmetric Val and Phe pseudopeptidic derivatives†
Abstract
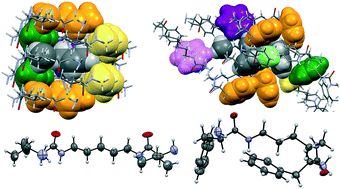
The self-assembly patterns of a family of C2-symmetric pseudopeptidic compounds have been studied in the crystalline state, analysing the influence of both the nature of the central spacer and that of the side chain of the amino acid on the observed features. The nature of the side chain of the component amino acid is the most important structural element defining the conformational and self-assembly patterns. The presence of an aromatic side chain, in the case of Phe derivatives, seems to play a key role in defining folded conformations with the aromatic side chain located over the central spacer. The formation of β-sheet-like stacks in crystals is predominant in Val derivatives but not in Phe-derived compounds. Side chains are strongly involved in intermolecular contacts, with isopropyl groups defining preferentially knob-into-hole interactions and aromatic groups of Phe pseudopeptides defining preferentially edge-to-face arrangements. NMR and CD data suggest that the conformational differences observed in the crystalline structures are also present in solution, in particular in polar solvents.


 Please wait while we load your content...
Please wait while we load your content...