Synthesis and structural characterization of transition metal dithiolene derivatives containing divalent metals as counter-cations†
Abstract
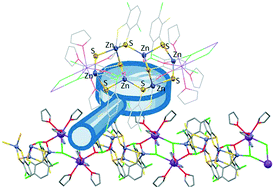
Direct reactions between HSC6H2Cl2SH, FeCl3·6H2O and divalent metal bases lead to the formation of the cation–anion metal–dithiolene complexes [Ca(H2O)3(OCMe2)4][Fe2(SC6H2Cl2S)4]·3OCMe2 and [Zn(DMF)6][Fe2(SC6H2Cl2S)4], as well as the molecular compound [Ba(OCMe2)6][Fe2(SC6H2Cl2S)4], instead of metal–dithiolene coordination polymers as compared with results previously obtained when alkali metals, under similar conditions, were used. An alternative synthetic route based on the reactions between K2[Fe2(SC6H2Cl2S)4] and ZnCl2·6H2O or NiCl2·6H2O was also evaluated, giving rise to the ion-pair compounds [Fe(H2O)2(THF)4][Ni(SC6H2Cl2S)2]2·4THF and [Fe(H2O)4(THF)2][Fe2(SC6H2Cl2S)4]·4THF and the coordination polymer {[K2(THF)8][Zn6(μ-Cl)2(SC6H2Cl2S)4(μ-κO:κO′-SC6H2Cl(ClO2)S)2]}n. It is interesting to remark that in the last compound the oxidation of one Cl substituent of a dithiolene ligand resulted in the formation of a Cl(O)2 group.


 Please wait while we load your content...
Please wait while we load your content...