Single-crystal GaN layer converted from β-Ga2O3 films and its application for free-standing GaN
Abstract
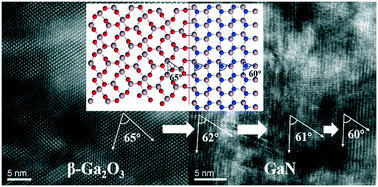
We have successfully obtained a hexagonal single crystal GaN layer with (002) orientation by nitridating β-Ga2O3 film under NH3 flow despite structural mismatch between β-Ga2O3 and hexagonal GaN. The conversion process of β-Ga2O3 to GaN has also been systematically investigated. The nitridated GaN layer shows a network structure without significant stress, which makes it very suitable to be used as a template for the epitaxial growth of high-quality GaN films. The GaN/Ga2O3 heterostructure can also be used to obtain free-standing GaN (FS-GaN) films by self-separation or chemical lift-off (CLO) process due to the selective etching of β-Ga2O3. Further investigation demonstrates the feasibility of the in situ growth of low-stress FS-GaN substrates by halide vapor phase epitaxy (HVPE).


 Please wait while we load your content...
Please wait while we load your content...