Light-controlled out-of-equilibrium assembly of cyclodextrins in an enzyme-mediated dynamic system†
Abstract
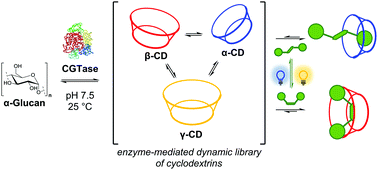
We show that the selective enzymatic synthesis of specific cyclodextrins can be modulated using light. We use enzyme-mediated dynamic combinatorial chemistry to generate a mixture of interconverting linear and cyclic α-1,4-glucans, and employ an azobenzene photoswitch as a template. Using UV or blue light to switch between photostationary states with different azobenzene cis/trans isomeric ratios, we can promote the out-of-equilibrium assembly of either α-cyclodextrin or β-cyclodextrin.


 Please wait while we load your content...
Please wait while we load your content...