Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly active and noble-metal-alternative hydrogenation catalysts prepared by dealloying Ni–Si intermetallic compounds†
Wiyanti F.
Simanullang
a,
Hiroshi
Itahara
 b,
Naoko
Takahashi
b,
Satoru
Kosaka
b,
Ken-ichi
Shimizu
b,
Naoko
Takahashi
b,
Satoru
Kosaka
b,
Ken-ichi
Shimizu
 ac and
Shinya
Furukawa
ac and
Shinya
Furukawa
 *ac
*ac
aInstitute for Catalysis, Hokkaido University, N21 W10, Kita-ku, Sapporo, 001-0021, Japan. E-mail: furukawa@cat.hokudai.ac.jp
bToyota Central Research & Development Labs, Inc., 41-1 Yokomichi Nagakute, Aichi 480-1192, Japan
cElements Strategy Initiative for Catalysts and Batteries, Kyoto University, Katsura, Kyoto 615-8520, Japan
First published on 29th October 2019
Abstract
Noble-metal-alternative Ni–Si catalysts more active than Pd in the hydrogen storage reaction were developed using a unique procedure, i.e., surface dealloying with hydrofluoric acid treatment. The combination of the structural analysis and the DFT calculation revealed a specific active site structure, a Ni cluster embedded in a SiO2 matrix, and its unprecedented role in the molecular conversion.
Hydrogenation is one of the most important chemical transformations in various systems such as industrial processes, pharmaceutical synthesis, and hydrogen storage.1 Noble metals such as Pt and Pd have been used as efficient catalysts for the hydrogenation of unsaturated hydrocarbons. However, because these noble metals are rare, unevenly distributed, and extremely expensive, the development of alternatives is strongly desired.2 As a non-noble metal, Ni has been used in various hydrogenation reactions involving, for example, aldehydes,3 nitroarenes,4 aromatics,5 and unsaturated fatty acids.6 However, severe reaction conditions such as high temperatures and hydrogen pressures are typically needed to attain sufficient reaction rates. Therefore, a drastic enhancement in the catalytic activity of Ni is a challenging but attractive approach to develop an efficient alternative to noble-metal catalysts.
Intermetallic compounds are receiving increasing attention as attractive catalyst materials because of their specific crystal structures, ordered atomic arrangements, and unique electronic states.7 Emerging research topics in this field include the use of intermetallic compounds as a platform to develop more efficient reaction environments via a chemical treatment.8–11 Two different well-ordered and atomically dispersed metal elements can be transformed into a composite with unconventional active sites via an appropriate chemical treatment.
In this study, we applied a “surface dealloying” methodology to a series of Ni-based intermetallic compounds such that only the surface structure was chemically modified, whereas the bulk structure was retained. During an attempt to develop an efficient hydrogenation catalyst based on surface dealloying, we discovered that some Ni–Si intermetallic compounds treated with a diluted aqueous solution of hydrofluoric acid (HF) exhibited outstanding catalytic performances in the hydrogenation of unsaturated hydrocarbons. In addition, a combination of X-ray photoelectron spectroscopy (XPS), scanning transmission electron microscopy (STEM) analysis, and density functional theory (DFT) studies suggested that Ni clusters partially embedded in the SiO2 matrix acted as highly active hydrogenation sites. Herein, we report a promising noble-metal-alternative material based on Ni with earth-abundant Si and a novel concept in catalyst preparation.
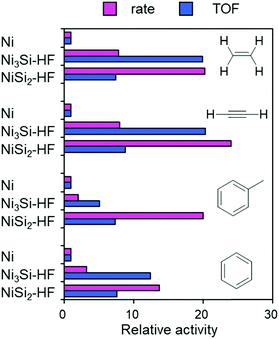
The procedure for catalyst preparation was very simple: a series of Ni-based intermetallic compounds prepared by arc melting (Ni3Si, Ni2Si, NiSi, NiSi2, Ni3Al, Ni3Ge, and Ni3Sn as fine powders; X-ray diffraction (XRD) patterns are shown in Fig. S1, ESI†) was added to a vigorously stirred aqueous solution of HF (typically 1.0 M) at 25 °C in air and the resultant solution was allowed to stand for 15 min, followed by three washings with deionized water. The thus-prepared catalysts were pre-reduced under flowing hydrogen at 400 °C and tested in the hydrogenation of ethylene as a model reaction (Table S1, ESI†). Fig. 1 shows the catalytic activities of these samples relative to that of pure Ni. Among the various materials, Ni3Si and NiSi2 showed remarkably high catalytic activities compared with Ni powder (a 20-fold higher turnover frequency (TOF) and reaction rate, respectively) and Ni/SiO2 (Ni nanoparticles supported on silica gel: 7–10 nm, Fig. S2, ESI†). Ni3Al–HF, which is a RANEY® Ni analog, did not demonstrate such a large enhancement. The catalytic activity did not change when pure Ni was treated with HF. No reaction occurred when Ni3Ge–HF or Ni3Sn–HF was used. These results indicate that the observed high catalytic activity is specific to the combination of Ni and Si.
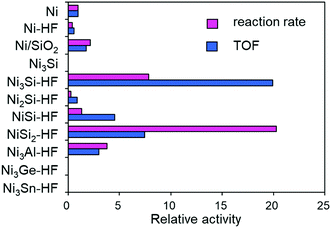 | ||
| Fig. 1 Relative catalytic activities of Ni-based materials in ethylene hydrogenation at 70 °C. Specific reaction rate per gram catalyst and TOF relative to those of pure Ni as unity are shown. | ||
We next characterized the structures of the Ni–Si catalysts before and after the HF treatment. The XRD patterns (Fig. S3, ESI†) and X-ray adsorption spectra (Fig. S4, ESI†) of the catalysts after HF treatment were almost identical to those of the untreated materials, indicating that the bulk structure was retained after the HF treatment. However, CO adsorption experiments revealed that the Ni dispersion substantially increased from zero depending on the concentration of HF (Fig. S5a, ESI†). These results suggest that Si species at the surface region were preferentially removed by the HF treatment, thus forming a Ni-rich surface. With increasing HF concentration, the Ni dispersion and the reaction rate initially increased and then plateaued (Fig. S5a and b, ESI†). The TOF reached a certain maximum at 1.0 ≤ [HF] ≤ 2.0 M for each Ni–Si intermetallic compound (Fig. S5c, ESI†). Details of the effect of [HF] are summarized in the ESI† (p. S25). SEM images of NiSi2 and NiSi2–HF revealed that the particle size and morphology did not change, while the surface became rough (Fig. S6, ESI†). This is consistent with the bulk and surface structures being retained and chemically modified, respectively.
Next, we tested the catalytic activities of Ni3Si–HF and NiSi2–HF in the hydrogenation of various unsaturated hydrocarbons such as acetylene, benzene, and toluene (Table S2, ESI†). Acetylene hydrogenation is the key process in purifying ethylene before polymerization.12 Benzene and toluene have attracted attention as promising hydrogen carriers;13,14 therefore, the development of an efficient catalyst for aromatic hydrogenation using non-noble metals is strongly desired. Fig. 2 shows the relative catalytic activity of these catalysts. In addition to their excellent ethylene hydrogenation performance, Ni3Si–HF and NiSi2–HF exhibited outstanding catalytic activities compared with that of Ni in acetylene hydrogenation. These catalysts also showed excellent aromatic hydrogenation performance (TOFs typically 10 or 20 times greater than that of Ni). NiSi2–HF gave the highest reaction rate in each reaction, likely because Ni was much more dispersed than in Ni3Si–HF (Fig. S5b, ESI†).
Given the aforementioned high reaction rates, we further studied the catalytic activity and stability of NiSi2–HF in aromatic hydrogenation under optimized conditions.
Fig. 3 shows the change in reactant conversion in the hydrogenation of benzene and toluene over NiSi2–HF, Pd black, and Ni catalysts. Here note that the Ni content in NiSi2–HF was 51 wt%. The pure Ni catalyst gave poor conversions less than 5% in both reactions. NiSi2–HF gave excellent conversions (ca. 93% for benzene and 91% for toluene) and retained them for at least 8 h. We also tested the catalytic activity of Pd black, in which the metal was slightly more dispersed than that in NiSi2–HF (Fig. 3 and Table S3, ESI†). Compared with Pd black, NiSi2–HF exhibited much higher and comparable conversions of benzene and toluene, respectively. NiSi2–HF also showed a 1.5-times higher TOF than Pd. The turnover number reached 5185 after 8 h on stream, which was much higher than that of Pd (3038, Table S3, ESI†). These results highlight that NiSi2–HF is a promising noble-metal-alternative material for hydrogen storage applications.
To consider the surface states of HF-treated catalysts, we conducted XPS analyses. Fig. 4a and b shows the Ni 2p3/2 and Si 2p XPS spectra of NiSi2 before and after the HF treatment and the subsequent H2 reduction.
The spectrum of NiSi2 shows emission peaks at 854.6 and 100.3 eV in the Ni 2p3/2 and Si 2p regions, respectively, consistent with reported values for zero-valent Ni and Si of NiSi2.15 An intense peak assignable to SiO2 was also observed at 103.6 eV,15 suggesting that the Si species at the surface were partially oxidized (Fig. 4c, left). After the HF treatment, the Ni 2p3/2 peak intensity increased, whereas the Si 2p intensity decreased, consistent with the removal of surface Si and an increase in Ni dispersion, as previously mentioned. Moreover, Si(0) species disappeared and two Ni species were newly observed at 852.8 and 856.0 eV, which were assignable to elemental Ni15 and NiO,16 respectively. These results indicate that Ni was separated from the parent intermetallic phase during the dealloying process by HF and that part of the Si remained as SiO2 (Fig. 4c). The presence of NiO after H2 reduction implies that some Ni species are fully embedded in the resulting SiO2 matrix, which hardly comes into contact with gaseous H2. Fig. 4d shows the bright field-STEM image of NiSi2–HF, where a specific layered structure is indeed observed (see also Fig. S7 for several viewpoints, ESI†). The elemental map in this field (Fig. S8, ESI†) revealed that each layer consisted of a number of small Ni clusters (2–5 nm) embedded in SiO2, SiO2, and NiSi2, which demonstrates the structural model shown in Fig. 4c. Thus, a specific surface structure consisting of Ni–SiO2 composites was obtained by applying the surface dealloying protocol. Given that Ni/SiO2 showed a TOF similar to that of bulk Ni, the high catalytic activities of Ni–Si–HF cannot be explained by a simple contact between the Ni and SiO2, as observed for supported nanoparticles. A similar trend in peak intensity was also observed for the XPS of Ni3Si and Ni3Si–HF (Fig. S9, ESI†). However, for Ni3Si, the surface of the parent Ni3Si was already segregated: the hard X-ray photoelectron spectra of Ni3Si revealed that the as-prepared Ni3Si had a thick SiO2 layer (>20 nm, Fig. S10, ESI†) at the surface. The presence of this thick SiO2 layer at the catalyst surface can explain why the untreated Ni3Si was inactive. The Ni3Si phase itself is known to be active toward hydrogenation:17,18 we recently reported that Ni3Si fine powder prepared by sodium melt synthesis (Ni3Si-p) exhibited high phase purity and a much less oxidized surface; hence, it exhibited a higher TOF than Ni in ethylene hydrogenation.17 However, emphasis should be placed on the observation that Ni3Si–HF still afforded a TOF more than two times that of Ni3Si-p under the same reaction conditions (Fig. S12, ESI†).
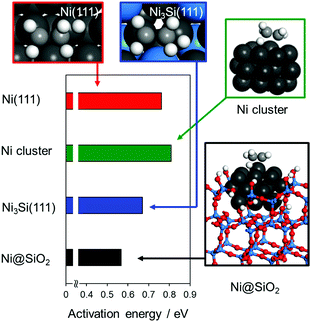
To clarify the role of surface structures in the observed enhancement in hydrogenation ability, we performed DFT calculations for the relevant Ni-based materials. The flat surfaces of Ni(111) and Ni3Si(111) were considered the standard active surfaces of Ni and Ni3Si-p, respectively. On the basis of the XPS and STEM analysis results, we considered a Ni cluster partially embedded in an SiO2 matrix to be the active site of Ni–Si–HF (hereafter, Ni@SiO2). In this study, we modeled a Ni20 cluster as a small truncated octahedron with or without a surrounding SiO2 matrix (Fig. S12, ESI†). For the Ni@SiO2 model, a cavity was made in a SiO2 framework with an appropriate size and OH terminations for the embedded Ni20 cluster to fit within the cavity. The closest contact between Ni and the lattice oxygen or OH groups ranged within 1.86–2.16 Å in the optimized structure (Fig. S12d, ESI†), which would provide moderate steric restriction or strain to the Ni cluster. Fig. 5 shows the activation energies associated with hydrogen attack at ethylene adsorbed onto the relevant Ni-based surfaces (detailed structures are shown in Fig. S13, ESI†). The Ni20 clusters gave an activation energy of 0.80 eV, which is similar to that on the Ni(111) surface (0.76 eV). This similarity indicates that the metal size weakly affects the reactivity, as reported in the literature.19 The Ni3Si(111) surface showed a lower activation energy of 0.67 eV, consistent with the catalytic activity of Ni3Si-p being higher than that of Ni. The lowest activation energy of 0.56 eV was observed for Ni@SiO2, where a substantial decrease in the energy barrier (0.24 eV) with the aid of the SiO2 matrix was observed. Thus, our calculation results could explain the experimental activity trend: Ni3Si–HF (Ni@SiO2) > Ni3Si-p > Ni ≈ Ni/SiO2. The substantial stabilization observed for Ni@SiO2 was attributed to the specific structure of the transition state: the active site of the Ni-atom-absorbing ethylene was uplifted compared with that of the free Ni cluster (Fig. 5 and Fig. S12, ESI†). Hydrogen attack to the upper-lying alkenyl carbon can be facilitated over the uplifted active sites, thereby stabilizing the transition state. This specific structure likely originates from the steric restriction provided by the surrounding SiO2 matrix: because the lateral displacement of Ni atoms is restricted, the displacement to the vertical direction could be amplified.
We also focused on the effect of the SiO2 matrix on the electronic state of Ni. However, the difference in the atomic charge of Ni between Ni20 and Ni@SiO2 was negligible (Table S4, ESI†). This result is consistent with the XPS analysis results: the surface Ni of Ni–Si–HF showed a binding energy identical to that of elemental Ni. The metal–support interaction is known to be weak for SiO2, unlike that for other common oxide supports such as TiO2 and Al2O3.20 This character may be one of the reasons why only Ni–Si–HF among the various investigated Ni–M–HF showed a specific improvement.
The Ni@SiO2 model we used is just one of the possible model structures. However, the important consideration is that our DFT model suggested an unprecedented strain effect on the catalytic activity. In addition, such a highly active reaction site could be constructed using the surface dealloying protocol, which is simple and easy. The obtained insights and catalyst design could also be applicable for supported nanoparticulate catalysts, which are expected to be much more efficient. We are currently focusing on developing supported Ni@SiO2 catalysts and will report another efficient system in the near future. Thus, our findings propose an innovative concept for catalyst design and would open a novel trend in catalysis research.
In summary, we prepared highly efficient catalysts for hydrogenation based on a novel concept of catalyst design, i.e., surface dealloying of intermetallic compounds. Compared with the conventional Ni catalysts, Ni3Si and NiSi2 treated with HF function as remarkably active hydrogenation catalysts for several unsaturated hydrocarbons, where a 10- to 20-fold increase in catalytic activity was achieved. NiSi2–HF exhibits excellent catalytic activity and stability in the hydrogenation of benzene and toluene. Emphasis should be placed on this catalyst being even more active than Pd in aromatic hydrogenation, indicating that the developed catalysts are efficient noble-metal-alternative materials for hydrogen storage and transport. The surface of Ni–Si is dealloyed by HF to form Ni–SiO2 composites, where Ni clusters are embedded in the SiO2 matrix. Steric restriction imposed on the Ni cluster by the SiO2 matrix enables stabilization of the hydrogenation transition state, which substantially lowers the activation barrier. The insights obtained in this study not only provide an efficient catalytic system but would also open a novel trend in catalyst design.
This work was partially supported by JSPS KAKENHI (Grant Numbers 17H01341 and 17H04965) and by “Elements Strategy Initiative to Form Core Research Center”. The HAXPES and XAFS analyses were performed with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2017A5071 and 2017B5070). Computation time was provided by the supercomputer systems in Institute for Chemical Research, Kyoto University.
Conflicts of interest
There are no conflicts to declare.References
- N. B. Johnson, I. C. Lennon, P. H. Moran and J. A. Ramsden, Acc. Chem. Res., 2007, 40, 1291–1299 CrossRef CAS PubMed.
- P. J. Chirik, Catalysis without Precious Metals, Wiley-VCH Verlag GmbH & Co. KGaA, 2010, pp. 83–110 DOI:10.1002/9783527631582.ch4.
- A. Reinsdorf, W. Korth, A. Jess, M. Terock, F. Klasovsky and R. Franke, ChemCatChem, 2016, 8, 3592–3599 CrossRef CAS.
- R. S. Downing, P. J. Kunkeler and H. vanBekkum, Catal. Today, 1997, 37, 121–136 CrossRef CAS.
- P. G. Savva, K. Goundani, J. Vakros, K. Bourikas, C. Fountzoula, D. Vattis, A. Lycourghiotis and C. Kordulis, Appl. Catal., B, 2008, 79, 199–207 CrossRef CAS.
- D. Jovanovic, R. Radovic, L. Mares, M. Stankovic and B. Markovic, Catal. Today, 1998, 43, 21–28 CrossRef CAS.
- S. Furukawa and T. Komatsu, ACS Catal., 2017, 7, 735–765 CrossRef CAS.
- M. Armbrüster, R. Schlögl and Y. Grin, Sci. Technol. Adv. Mater., 2014, 15, 034803 CrossRef PubMed.
- S. Kameoka, S. Wakabayashi, E. Abe and A. P. Tsai, Catal. Lett., 2016, 146, 1309–1316 CrossRef CAS.
- S. Furukawa, A. Tsuchiya, Y. Kojima, M. Endo and T. Komatsu, Chem. Lett., 2016, 45, 158–160 CrossRef CAS.
- S. Shoji, X. Peng, T. Imai, P. S. Murphin Kumar, K. Higuchi, Y. Yamamoto, T. Tokunaga, S. Arai, S. Ueda, A. Hashimoto, N. Tsubaki, M. Miyauchi, T. Fujita and H. Abe, Chem. Sci., 2019, 10, 3701–3705 RSC.
- A. Borodzinki, Catal. Rev., 2006, 48, 91–144 CrossRef.
- R. B. Biniwale, S. Rayalu, S. Devotta and M. Ichikawa, Int. J. Hydrogen Energy, 2008, 33, 360–365 CrossRef CAS.
- Y. Okada, E. Sasaki, E. Watanabe, S. Hyodo and H. Nishijima, Int. J. Hydrogen Energy, 2006, 31, 1348–1356 CrossRef CAS.
- Y. Cao, L. Nyborg and U. Jelvestam, Surf. Interface Anal., 2009, 41, 471–483 CrossRef CAS.
- M. A. Peck and M. A. Langell, Chem. Mater., 2012, 24, 4483–4490 CrossRef CAS.
- H. Itahara, W. F. Simanullang, N. Takahashi, S. Kosaka and S. Furukawa, Inorg. Chem., 2019, 58, 5406–5409 CrossRef CAS PubMed.
- P. Ryabchuk, G. Agostini, M.-M. Pohl, H. Lund, A. Agapova, H. Junge, K. Junge and M. Beller, Sci. Adv., 2018, 4, eaat0761 CrossRef PubMed.
- C.-K. Tsung, J. N. Kuhn, W. Huang, C. Aliaga, L.-I. Hung, G. A. Somorjai and P. Yang, J. Am. Chem. Soc., 2009, 131, 5816–5822 CrossRef CAS PubMed.
- S. Furukawa, K. Ozawa and T. Komatsu, RSC Adv., 2013, 3, 23269–23277 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc07862b |
| This journal is © The Royal Society of Chemistry 2019 |