Open Access Article
Open Access ArticleCation–π interactions secure aggregation induced emission of planar organic luminophores†
Kaspars
Leduskrasts
 ,
Artis
Kinens
,
Artis
Kinens
 and
Edgars
Suna
and
Edgars
Suna
 *
*
Latvian Institute of Organic Synthesis, Aizkraukles 21, Riga, LV-1006, Latvia. E-mail: edgars@osi.lv
First published on 4th October 2019
Abstract
The use of non-covalent intermolecular π+–π interactions between quaternary pyridinium or imidazolium cations and aromatic π systems is an efficient approach to achieve AIE in planar purely organic luminophores.
Organic luminophores that are highly emissive in dilute solutions frequently experience decrease or even loss of the emission upon aggregation. This phenomenon is known as aggregation-caused quenching (ACQ).1 The ACQ is a major hurdle in many practical applications, which require emission in the aggregated state (thin films, polymer matrices, etc.) or in the solid state.2–6 In 2001 Tang discovered aggregation induced emission luminogens (AIEgens) that featured increased emission in the solid state as compared to that in dilute solution.7 Since then AIEgens have found ample applications in various optoelectronic devices such as OLEDs,8 organic field-effect transistors5,9 and photovoltaic devices10 as well as in artificial photosynthesis11 and photon refining.12 AIEgens have also been widely used as chemical sensors,13 biosensors,14 stimuli sensors15 and as luminescent probes in biomedical applications.16
The working principle underlying the emission of AIEgens is proposed to be a restriction of intramolecular motions (RIM), vibrations (RIV) and rotations (RIR) to minimize the non-radiative dissipation of exciton energy.16b,17 In addition, the design of AIEgens also frequently features out-of-plane twisting of aromatic subunits and incorporation of a steric bulk to avoid the detrimental intermolecular π–π stacking interactions between planar aromatic luminophores, which results in the ACQ effect. These structural requirements usually lead to a relatively complex design of purely organic AIEgens.16a,b,18 Clearly, a new type of interaction that would help to achieve AIE in structurally simple purely organic molecules is highly desired for the development of AIEgens.
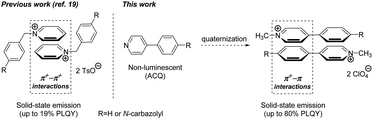
Recently we have demonstrated that non-covalent intermolecular interactions between pyridinium (Py+) cations (π+–π+ interactions) leads to a solid state luminescence (SSL) with up to 19% PLQY (see Fig. 1).19 In this study we report on a further development of the conceptual approach to achieve AIE. Specifically, we show herein that a mechanistically related interaction between quaternary Py+ or imidazolium (Im+) cations and aromatic π systems (π+–π interactions) results in remarkable SSL (up to 80% PLQY). Furthermore, the quaternization of nitrogen atoms turns on the SSL even in planar heteroaromatic molecules (Fig. 1). The performed DFT calculations suggest that the observed SSL results from intermolecular charge transfer (ICT) between heteroaromatic (Py+ and Im+) cations and aromatic π systems.
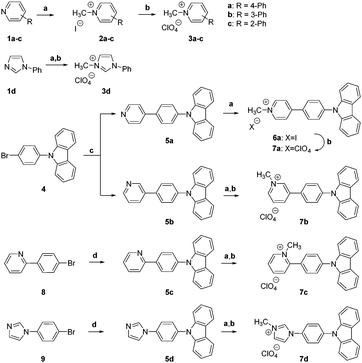
Py+ and Im+ perchlorates 3a–d were obtained by N-alkylation of the commercially available heterocycles 1a–d with MeI, followed by the exchange of iodide for perchlorate (Fig. 2). Pyridines 5a,b were synthesized from the commercially available 4 and pyridine boronic acids under Suzuki reaction conditions. Subsequent N-alkylation with MeI was followed by iodide-to-perchlorate exchange to afford Py+ salts 7a,b. The synthesis of heterocycles 5c and 5d required Cu(I)-catalyzed N-arylation of carbazole by the commercially available bromides 8 and 9, respectively. A subsequent N-alkylation and anion exchange sequence provided 7c,d (Fig. 2). All heteroaromatic quaternary salts 3a–d, 6a and 7a–d were crystalline materials.
UV-vis spectra of all synthesized quaternary salts and parent heterocycles were measured in MeCN solutions (at ca. 10−5 mol L−1) at room temperature and under an ambient atmosphere. For analysis of absorption spectra, see Table 1 and the ESI,† page S16. Heteroarenes 1a–d did not show any measurable emission in solution and in the solid state with <0.1% photoluminescence quantum yields (PLQYs). The lack of emission for 1a–d was not surprising because these molecules do not possess any of the traditional luminophore moieties such as carbazole. Alkylation of 1a with MeI afforded Py+ iodide 2a, which displayed a broad non-structured emission band at λmax = 372 nm in MeCN solution with 36.8% PLQY (entry 1, Table 1).20 Unfortunately, Py+ salt 2a was non-emissive in the solid state, and the lack of SSL for Py+ iodides has been reported earlier by Fossey.21 We were pleased to find that a simple exchange of iodide 2a to perchlorate 3a enabled high SSL (λmax = 346 nm, 52.7% PLQY), while retaining the emission in MeCN solution (broad emission band at λmax = 372 nm with 37.9% PLQY; entry 2). Isomeric perchlorates 3b,c also displayed observable emission in solution (at λmax = 390 nm and 380 nm with PLQYs of 21.1% and 3.5%, respectively) and high SSL with PLQYs of 42.1% (λmax = 350 nm) and 39.1% (λmax = 341 nm), respectively (entries 3 and 4). Decreased PLQY for 3c both in solution and in the solid state could be possibly attributed to the decreased conjugation due to the presence of a N-methyl substituent. We were also pleased to find that SSL can be achieved using cationic heteroaromatic subunits other than Py+ salts. Thus, Im+ perchlorate 3d showed luminescence both in solution (λmax = 380 nm, 16.1% PLQY) and in the solid state (λmax = 315 nm, 19.8% PLQY; entry 5). Notably, all perchlorates 3a–d featured AIE properties as evidenced by higher PLQY in the solid state as compared to solution (αAIE up to 11.2; see Table 1). The observed SSL levels for 3a–d (up to 53% PLQY for 3a) are remarkable given the structural simplicity of salts 3a–d and the absence of a luminophore moiety in their structure.
| Entry | Compound | λ abs, nm | Solution λem, nm | Solid λem, nm | Solution, ϕ (%) | Solid, ϕ (%) | α AIE |
|---|---|---|---|---|---|---|---|
| 1 | 2a | 247, 293 | 372 | — | 36.8 | <0.1 | <0.1 |
| 2 | 3a | 231, 294 | 372 | 346 | 37.9 | 52.7 | 1.4 |
| 3 | 3b | 237, 257, 292 | 390 | 350 | 21.2 | 42.1 | 2.0 |
| 4 | 3c | 239, 282 | 380 | 341 | 3.5 | 39.1 | 11.2 |
| 5 | 3d | 235 | 353 | 315 | 16.1 | 19.8 | 1.2 |
| 6 | 5a | 238, 292, 322 | 442 | 371, 387, 407 | 73.1 | 5.7 | 0.1 |
| 7 | 5b | 240, 292, 338 | 408 | 368 | 46.5 | 0.6 | <0.1 |
| 8 | 5c | 240, 292, 315 | 416 | 378, 436 | 74.6 | 16.8 | 0.2 |
| 9 | 5d | 240, 292, 326, 339 | 347, 361 | 371 | 33.2 | 17.8 | 0.5 |
| 10 | 6a | 237, 282, 376 | — | 473 | <0.1 | 0.40 | >4 |
| 11 | 7a | 239, 282, 379 | — | 489 | <0.1 | 79.8 | >800 |
| 12 | 7b | 236, 289, 339 | — | 446 | <0.1 | 72.2 | >700 |
| 13 | 7c | 237, 279, 337 | — | 465 | <0.1 | 46.9 | >470 |
| 14 | 7d | 241, 291, 336 | 498 | 369 | 1.6 | 11.0 | 6.9 |
Next, we incorporated carbazole into AIEgens 3a–d to increase their luminescence efficiency. Highly intense SSL was observed for perchlorates 7a (79.8% PLQY at λmax = 489 nm), 7b (72.2% PLQY at λmax = 446 nm) and 7c (46.9% PLQY at λmax = 465 nm; entries 11–13). Strikingly, Py+ perchlorates 7a–c were non-emissive in MeCN solution with PLQY < 0.1%. The observed up to 800-fold increase of PLQY in the solid state as compared to that in the MeCN solution confirms the AIE nature of 7a–c. Perchlorate 7d demonstrated slightly reduced SSL (11.0% PLQY at λmax = 498 nm; entry 14) vs. parent 3d (entry 5). In contrast to perchlorate 7a, the corresponding iodide 6a showed luminescence neither in the MeCN solution nor in the solid state (entry 10). The observed lack of the luminescence for iodides 2a and 6a demonstrates the importance of the counter-ion in the quaternized luminophores. Finally, the luminescence properties of parent 5a–d were also determined. All non-charged heterocycles 5a–d featured high emission intensity in MeCN solutions (entries 6–9) with 5a showing the highest PLQY (73.1%). In contrast, considerably reduced SSL was observed for 5a–d. Thus, 5b showed up to 80-fold decrease of PLQY in the solid state as compared to the MeCN solution (entry 7). Evidently, the reduced SSL is a result of the ACQ effect in the highly planar luminophores 5a–d, which experience intermolecular π–π aromatic interactions leading to decay of the excited-state energy via non-radiative energy transfer.22 In contrast, ACQ does not affect the structurally related planar salts 7a–d as evidenced by the pronounced AIE properties of these luminophores. Furthermore, the change in structured emission for 5a in the solid state to a broad featureless solid state emission of perchlorate 7a indicates distinct emission mechanisms for 5a and 7a.
The AIE properties of perchlorates 7a–d were further corroborated by the observed correlation between the emission and formation of aggregates (see the ESI,† page S27, for details). In addition, lack of emission for 7a in solution and the frozen DMSO matrix (solid amorphous state) speaks against the RIR effect as the origin of the AIE properties of quaternary salts 3a–d and 7a–d (see the ESI,† page S29, for details). A notable feature of perchlorates 3a–d and 7d is the SSL in the UV region (315–369 nm; entries 2–5 and 14), a property that has been rarely observed for AIE materials.23 The attachment of the carbazole moiety to 3a–d resulted in a substantial (54–143 nm) red-shift of the SSL. Hence, the introduction of electron donating moieties in 3a–d shifts the emission maxima, allowing for direct control of the emission wavelength.
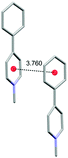
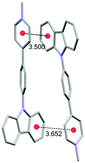
Single crystal X-ray analysis of 3a, 6a and 7a provided important insight into the intermolecular interactions that lead to the SSL (Table 2). In a crystal lattice, salts 3a, 6a and 7a showed close interactions between charged Py+ cations and π systems (π+–π interactions), which ranged from 3.500 Å (6a) to 3.760 Å (3a; Table 2). However, the distance between centroids does not correlate with the emission efficiency. Thus, very low PLQY (0.4%) was observed for 6a in the solid state despite the short distances for π+–π interactions (3.500 Å and 3.652 Å). Salt 7a also featured short distance between Py+ cations and carbazole π-systems (3.525 Å), whereas the corresponding distance in 3a was longer (3.760 Å). Nevertheless, both 3a and 7a were superior to 6a with respect to the SSL efficiency (52.7% and 79.8% PLQY, respectively). The interacting Py+ and carbazole planar rings are nearly parallel in 6a and 7a (5.65° and 3.73° angles between the planes of heterocycles, respectively), and the angle between the Py+ subunit and arene ring in 3a is 19.1°. Despite the apparent similarity between the packing mode of 6a and 7a, the remarkable difference in the solid state PLQY suggests that the iodide ion quenches the emission in 6a by mechanisms other than steric or electronic hindrance of the π+–π interactions.
Additional support for the relationship between π+–π interactions (involving Py+ cations and carbazole π systems) and the SSL was obtained by time-dependent density functional theory (TDDFT) calculations of the emission spectra for 7a at the B3LYP/6-31G(d) level of theory (see the ESI,† page S33). As Py+ perchlorate 7a did not display emission in solution, the emission spectra of 7a were calculated using geometry from the crystalline state. Furthermore, only singlet states Sn were used for calculations because oxygen quenching experiments in the suspensions of 7a did not show any emission intensity change, thus providing evidence against triplet state emission.
First, the feasibility of intramolecular CT character of the SSL in cation 7a was examined at the single molecule level. The calculated emission spectra (Fig. 3A) displayed two distinct emission peaks at λmax = 341 nm and 740 nm with high oscillatory force (f = 0.3387 and f = 0.3063; see Fig. 3A). Molecular orbitals (MO) associated with the electronic transitions were localized in the carbazole moiety (HOMO) and in the Py+ moiety with lesser intensity occupying also the phenylene linker (LUMO; see Fig. 3C and the ESI,† page S33, for the representation of frontier MO). The emission peak at λmax = 341 nm corresponded to transition from HOMO−4 to the LUMO and from the HOMO to LUMO+3. The emission peak at λmax = 740 nm corresponded to transition from the HOMO to LUMO (see Fig. 3B and the ESI†). Notably, the calculated emission peaks did not match the experimentally observed one at λmax = 489 nm (Table 1, entry 11). Consequently, an intramolecular CT apparently does not account for the observed SSL of 7a.
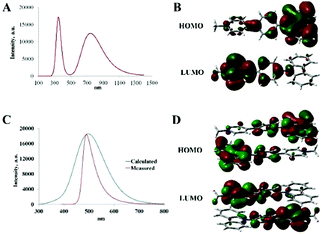 | ||
| Fig. 3 (A) Calculated UV-Vis spectrum of 7a (single molecule). (B) Frontier MO for 7a (single molecule). (C) Calculated UV-Vis spectrum of the dimer of 7a. (D) Frontier MO for the dimer of 7a. | ||
Next, the emission spectra for the symmetric dimer of 7a were calculated, resulting in a broad emission peak at λmax = 495 nm with a large oscillatory force (f = 0.3784; Fig. 3C). MO associated with the electronic transitions was localized in two regions of the dimer: the HOMO was located on the carbazole moiety, whereas the LUMO was mostly localized on the Py+ moiety with lesser intensity occupying also the phenylene linker (see Fig. 3C and the ESI† for the representation of the frontier MO). Calculations show that multiple CT (from HOMO−3 to the LUMO, from HOMO−2 to LUMO+1, from HOMO−1 to LUMO and from HOMO to LUMO+1; see the ESI†) are responsible for the emission at λmax = 495 nm. Such a type of symmetric through-space CT has been reported for the SSL.24 Notably, the calculated emission at λmax = 495 nm for the dimer of 7a was consistent with that observed experimentally (λmax = 489 nm; entry 11). However, the observed SSL peak for 7a was narrower compared to the calculated one. This difference could be possibly attributed to the additional stabilizing interactions in the crystal lattice for 7a. Consequently, the TDDFT calculations provide evidence that an emissive intermolecular through-space CT band is responsible for the SSL of cationic perchlorates 3a–d and 7a–d.
In summary, a series of highly emissive planar AIEgens has been designed by utilizing non-covalent intermolecular π+–π interactions between Py+ or Im+ cations and aromatic π systems. Structurally simple salts 3a–d lacking any conventional luminophore moiety demonstrated high SSL (up to 53% PLQY) in the UV region (315–350 nm). The introduction of electron donating moieties in 3a–d resulted in bathochromic shifts of the emission maxima, thus allowing for direct control of emission wavelength. Carbazole-containing perchlorates 7a–d also demonstrated a remarkable (up to 800-fold) increase of PLQY as compared to that in MeCN solution. The presence of iodide ions led to the quenching of the SSL. Single crystal X-ray analyses confirm the presence of non-covalent intermolecular interactions between Py+ or Im+ cations and aromatic π systems in the crystal state of AIEgens 3a and 7a. TDDFT calculations provide strong evidence that the observed SSL of 3a–d and 7a–d is a result of intermolecular π+–π interactions that generate through-space CT bands in the crystal state. The use of the non-covalent π+–π interactions in the design of AIEgens is a complementary approach to routinely used means to achieve AIE properties and to avoid the ACQ effect. We believe that our study will expand the scope of structural motifs that previously could not be used due to the ACQ and will open a new avenue for the rational design of AIEgens.
This work was funded by ERDF project No. 1.1.1.1/18/A/063. We thank Dr S. Belyakov and Dr A. Mishnev for X-ray crystallographic analysis and P. Dimitrijevs for DLS measurements.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) A. S. Klymchenko, Acc. Chem. Res., 2017, 50, 366 CrossRef CAS PubMed; (b) S. A. Jenekhe and J. A. Osaheni, Science, 1994, 265, 765 CrossRef CAS PubMed.
- R. B. Thompson, Fluorescence Sensors and Biosensors, CRC/Taylor & Francis, Boca Raton, 2006 Search PubMed.
- C. D. Geddes and J. R. Lakopwicz, Advanced Concepts in Fluorescence Sensing, Springer, New York, 2005 Search PubMed.
- A. Buckley, Organic light-emitting diodes (OLEDs): materials, devices and applications, Woodhead Publishing, Oxford, 2013 Search PubMed.
- S. G. Surya, H. N. Raval, R. Ahmad, P. Sonar, K. N. Salama and V. R. Rao, TrAC, Trends Anal. Chem., 2019, 111, 27 CrossRef CAS.
- E. Fresta and R. D. Costa, J. Mater. Chem. C, 2017, 5, 5643 RSC.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740 RSC.
- (a) Z. Ning, Z. Chen, Q. Zhang, Y. Yan, S. Qian, Y. Cao and H. Tian, Adv. Funct. Mater., 2007, 17, 3799 CrossRef CAS; (b) J. Huang, N. Sun, Y. Dong, R. Tang, P. Lu, P. Cai, Q. Li, D. Ma, J. Qin and Z. Li, Adv. Funct. Mater., 2013, 23, 2329 CrossRef CAS.
- (a) Z. Zhao, Z. Li, J. W. Y. Lam, J.-L. Maldonado, G. Ramos-Ortiz, Y. Liu, W. Yuan, J. Xu, Q. Miao and B. Z. Tang, Chem. Commun., 2011, 47, 6924 RSC; (b) M. P. Aldred, G.-F. Zhang, C. Li, G. Chen, T. Chen and M.-Q. Zhu, J. Mater. Chem. C, 2013, 1, 6709 RSC.
- (a) B. Mi, Y. Dong, Z. Li, J. W. Y. Lam, M. Haussler, H. H. Sung, H. S. Kwok, Y. Dong, I. D. Williams, Y. Liu, Y. Luo, Z. Shuai, D. Zhu and B. Z. Tang, Chem. Commun., 2005, 3583 RSC; (b) Y. Li, Z. Li, Y. Wang, A. Compaan, T. Ren and W.-J. Dong, Energy Environ. Sci., 2013, 6, 2907 RSC.
- (a) M. Zhang, X. Yin, T. Tian, Y. Liang, W. Li, Y. Lan, J. Li, M. Zhou, Y. Ju and G. Li, Chem. Commun., 2015, 51, 10210 RSC; (b) Y. Zeng, P. Li, X. Liu, T. Yu, J. Chen, G. Yang and Y. Li, Polym. Chem., 2014, 5, 5978 RSC.
- (a) P. Duan, D. Asthana, T. Nakashima, T. Kawai, N. Yanai and N. Kimizuka, Faraday Discuss., 2017, 196, 305 RSC; (b) L. Li, Y. Zeng, T. Yu, J. Chen, G. Yang and Y. Li, ChemSusChem, 2017, 10, 4610 CrossRef CAS PubMed.
- (a) X. Wang, J. Hu, T. Liu, G. Zhang and S. Liu, J. Mater. Chem., 2012, 22, 8622–8628 RSC; (b) Y. Chen, W. Zhang, Y. Cai, R. T. K. Kwok, Y. Hu, J. W. Y. Lam, X. Gu, Z. He, Z. Zhao, X. Zheng, B. Chen, C. Gui and B. Z. Tang, Chem. Sci., 2017, 8, 2047 RSC.
- X. Xue, Y. Zhao, L. Dai, X. Zhang, X. Hao, C. Zhang, S. Huo, J. Liu, C. Liu, A. Kumar, W. Q. Chen, G. Zou and X. J. Liang, Adv. Mater., 2014, 26, 712 CrossRef CAS PubMed.
- (a) J. Q. Shi, W. J. Zhao, C. H. Li, Z. P. Liu, Z. S. Bo, Y. P. Dong, Y. Q. Dong and B. Z. Tang, Chin. Sci. Bull., 2013, 58, 2723 CrossRef CAS; (b) Q. Qi, X. Fang, Y. Liu, P. Zhou, Y. Zhang, B. Yang, W. Tian and S. X.-A. Zhang, RSC Adv., 2013, 3, 16986 RSC.
- (a) Y. Tang and B. Z. Tang, Principles and Applications of Aggregation-Induced Emission, Springer International Publishing, New York, 2019 CrossRef; (b) J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718 CrossRef CAS PubMed.
- (a) C. Yuan, S. Saito, C. Camacho, T. Kowalczyk, S. Irle and S. Yamaguchi, Chem. – Eur. J., 2014, 20, 2193 CrossRef CAS PubMed; (b) L. Yao, S. Zhang, R. Wang, W. Li, F. Shen, B. Yang and Y. Ma, Angew. Chem., Int. Ed., 2014, 53, 2119 CrossRef CAS PubMed; (c) J. Liu, X. Zhang, X. Lu, P. He, L. Jiang, H. Dong and W. Hu, Chem. Commun., 2013, 49, 1199 RSC.
- (a) X. Wang, S. Wang, J. Lv, S. Shao, L. Wang, X. Jing and F. Wang, Chem. Sci., 2019, 10, 2915 RSC; (b) S.-K. Kim, S.-Y. Oh and J.-W. Park, Thin Solid Films, 2008, 517, 1349 CrossRef CAS.
- K. Leduskrasts and E. Suna, RSC Adv., 2019, 9, 460 RSC.
- W. Chen, S. A. Elfeky, Y. Nonne, L. Male, K. Ahmed, C. Amiable, P. Axe, S. Yamada, T. D. James, S. D. Bull and J. S. Fossey, Chem. Commun., 2011, 47, 253 RSC.
- I. Richter, M. R. Warren, J. Minari, S. A. Elfeky, W. Chen, M. F. Mahon, P. R. Raithby, T. D. James, K. Sakurai, S. J. Teat, S. D. Bull and J. S. Fossey, Chem. – Asian J., 2009, 4, 194 CrossRef CAS PubMed.
- See the ESI,† page S23, for further discussions.
- (a) C.-Q. Ye, L.-W. Zhou, C.-B. Fan, G.-L. Dai, X.-M. Wang, X.-T. Tao, P.-Y. Tang and W.-M. Su, ChemistrySelect, 2019, 4, 2044 CrossRef CAS; (b) Z. Gan, M. Meng, Y. Di and S. Huang, New J. Chem., 2016, 40, 1970 RSC.
- X.-H. Jin, C. Chen, C.-X. Ren, L.-X. Cai and J. Zhang, Chem. Commun., 2014, 50, 15878 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, photo physical properties, X-ray crystallographic data (CIF files), DFT calculations, and 1H and 13C NMR spectra. CCDC 1949701–1949703. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc06829e |
| This journal is © The Royal Society of Chemistry 2019 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: