A fluorinated bihydrazide conjugate for activatable sensing and imaging of hypochlorous acid by 19F NMR/MRI†
Abstract
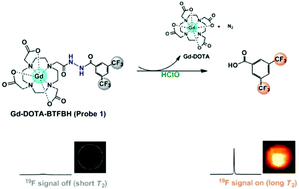
Hypochlorous acid (HClO) is one of the most important reactive oxygen species (ROS) and plays a vital role in many physiological and pathological processes. The comprehensive exploration of mechanistic details and the potential clinical translation necessitate the development of reliable probes for prompt and accurate detection of HClO in complex biological environments. Herein we report a fluorinated bihydrazide conjugate as a 19F NMR/MRI probe with a “turn-on” character for the detection of HClO. This probe could selectively respond to HClO, leading to a significant recovery of 19F signals for 19F NMR/MRI. Activatable sensing and imaging of HClO were achieved with SMMC-7721 cells and nude mice, which demonstrates that this small molecular conjugate could serve as a selective probe for real-time sensing and imaging of HClO in biological systems.


 Please wait while we load your content...
Please wait while we load your content...